Exam 1: Covalent Bonding and Shapes of Molecules
Exam 1: Covalent Bonding and Shapes of Molecules118 Questions
Exam 2: Alkanes and Cycloalkanes101 Questions
Exam 3: Stereoisomerism and Chirality95 Questions
Exam 4: Acids and Bases94 Questions
Exam 5: Alkenes: Bonding, Nomenclature, and Properties88 Questions
Exam 6: Reactions of Alkenes97 Questions
Exam 7: Alkynes100 Questions
Exam 8: Haloalkanes, Halogenation, and Radical Reactions75 Questions
Exam 9: Nucleophilic Substitution and Beta-Elimination104 Questions
Exam 10: Alcohols102 Questions
Exam 11: Ethers, Epoxides, and Sulfides102 Questions
Exam 12: Infrared Spectroscopy74 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy99 Questions
Exam 14: Mass Spectrometry75 Questions
Exam 15: An Introduction to Organometallic Compounds73 Questions
Exam 16: Aldehydes and Ketones122 Questions
Exam 17: Carboxylic Acids76 Questions
Exam 18: Functional Derivatives of Carboxylic Acids117 Questions
Exam 19: Enolate Anions and Enamines96 Questions
Exam 20: Dienes, Conjugated Systems, and Pericyclic Reactions75 Questions
Exam 21: Benzene and the Concept of Aromaticity77 Questions
Exam 22: Reactions of Benzene and Its Derivatives109 Questions
Exam 23: Amines92 Questions
Exam 24: Catalytic Carbon-Carbon Bond Formation84 Questions
Exam 25: Carbohydrates69 Questions
Exam 26: Lipids65 Questions
Exam 27: Amino Acids and Proteins77 Questions
Exam 28: Nucleic Acids58 Questions
Exam 29: Organic Polymer Chemistry68 Questions
Select questions type
What is the approximate value of the length of the C≡C bond in ethyne, HC≡CH?
(Multiple Choice)
4.9/5  (39)
(39)
The following species forms during an organic reaction. 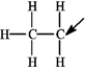 The formal charge on the carbon atom indicated by the arrow is +1.
The formal charge on the carbon atom indicated by the arrow is +1.
(True/False)
4.8/5  (42)
(42)
Which of the following molecules has a molecular dipole moment?
(Multiple Choice)
4.9/5  (37)
(37)
Which atomic orbitals overlap to form the carbon-hydrogen σ bonding molecular orbitals of ethane, CH3CH3?
(Multiple Choice)
4.8/5  (29)
(29)
Provide a neatly drawn figure to show the atomic orbitals that overlap to form each of the bonds in ethyne (acetylene, HC≡CH). Label each bond (e.g., C-H σ bond) and indicate which atomic orbitals contribute to this bond (e.g., C 2sp3 + H 1s).
(Essay)
4.8/5  (32)
(32)
Which of the following resonance structures makes the largest contribution to the structure of [H2CCHO] −? ![Which of the following resonance structures makes the largest contribution to the structure of [H<sub>2</sub>CCHO] <sup>−</sup>?](https://storage.examlex.com/TB7078/11ead7c8_9ddb_2fc6_84b0_e38b045e89d7_TB7078_00.jpg)
(Multiple Choice)
4.8/5  (46)
(46)
What is the approximate C−C−C bond angle in propene, CH3CH=CH2?
(Multiple Choice)
4.9/5  (26)
(26)
Draw bond-line structures of all of the tertiary (3°) alcohols that have the formula C6H14O.
(Essay)
4.9/5  (30)
(30)
Provide a neatly drawn figure to show the atomic orbitals that overlap to form each of the bonds in ethene (ethylene, H2C=CH2). Label each bond (e.g., C-H σ bond) and indicate which atomic orbitals contribute to this bond (e.g., C 2sp3 + H 1s).
(Essay)
4.8/5  (35)
(35)
The energy released on the addition of an electron to an atom or a molecule is called _____.
(Multiple Choice)
4.9/5  (34)
(34)
What is the approximate strength of the C−C bond of ethane?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following molecules has a molecular dipole moment?
(Multiple Choice)
4.8/5  (40)
(40)
Which atomic orbitals overlap to form the carbon-carbon σ and π bonding molecular orbitals of ethene, H2C=CH2?
(Multiple Choice)
4.7/5  (37)
(37)
Draw bond-line structures of all of the ketones that have the formula C5H10O.
(Essay)
4.9/5  (38)
(38)
Which atomic orbitals overlap to form the C=O bond of acetone, (CH3)2C=O?
(Multiple Choice)
4.7/5  (33)
(33)
What is the approximate value of the length of the C=C bond in ethane, CH2=CH2?
(Multiple Choice)
4.8/5  (34)
(34)
Circle all of the sp hybridized atoms in the following molecular structure. 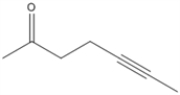
(Essay)
4.8/5  (38)
(38)
Showing 61 - 80 of 118
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)