Exam 5: Thermochemistry: Energy Changes in Reactions
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Using the following data for water, determine the final temperature when 100 g of ice at -10°C is heated with 350 kJ of energy. Boiling point 373 Melting point 273 Enthalpy of vaporization 2,260/ Enthalpy of fusion 334/ Specific heat capacity (solid) 2.11/(\cdot) Specific heat capacity (liquid) 4.18/(\cdot) Specific heat capacity (gas) 2.08/(\cdot)
(Multiple Choice)
4.8/5  (33)
(33)
The enthalpy of combustion of table sugar (sucrose, C12H22O11, 342.3 g/mol) is -5,643 kJ/mol. What is the food value (Cal/g) of sucrose? (1 Cal = 4.184 kJ)
(Multiple Choice)
4.8/5  (31)
(31)
In terms of the enthalpy of formation, which of the following compounds is the most unstable compared to its elements under standard conditions?
(Multiple Choice)
4.8/5  (38)
(38)
A heating curve for some substance is shown below. Which of the line segments (I-V) represents heating of the liquid? 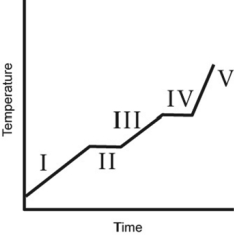
(Multiple Choice)
4.9/5  (43)
(43)
An expanding gas does 175 kJ of work on its surroundings at a constant pressure of 5.55 atm. If the gas initially occupied 125 mL, what is the final volume of the gas? (101.3 J = 1 L atm)
(Multiple Choice)
4.8/5  (45)
(45)
You heat a cup of coffee in a microwave oven. It absorbs 40 kJ of energy from the microwave, and the volume expands. Which one of the following statements correctly describes the relationship between the change in enthalpy H and the change in internal energy E of the coffee?
(> means greater than, < means less than)
(Multiple Choice)
4.9/5  (33)
(33)
The diagram below shows three ion pairs: (a) a doubly charged anion and cation, (b) a singly charged anion and cation, and (c) two doubly charged anions.
(I) Label each pair to identify the electrostatic interaction as attractive or as repulsive.
(II) Which pair has the largest electrostatic interaction energy that is positive?
(III) Which pair has the largest electrostatic interaction energy that is negative?
(IV) Which pair has the smallest electrostatic interaction energy? 
(Essay)
4.9/5  (46)
(46)
Thermochemistry is the study of how ________ is produced and consumed during chemical reactions.
(Multiple Choice)
4.9/5  (38)
(38)
A balanced reaction equation with a H value is called ________
(Multiple Choice)
4.8/5  (42)
(42)
From year to year, the water level in a lake varies, as shown below. At which time is the potential energy of the water behind the dam greatest?
(Multiple Choice)
4.7/5  (34)
(34)
Lightweight camping stoves typically use a mixture of C5 and C6 hydrocarbons, which is called white gas. Assuming white gas is 100% C5H12 with an enthalpy of combustion of -3,550 kJ/mol, how many grams of it must be used to heat 1.50 L of water from 20°C to 80°C? Assume all the energy is used to heat the water.
(Multiple Choice)
4.7/5  (44)
(44)
When 1.14 g of octane (molar mass = 114 g/mol) reacts with excess oxygen in a constant volume calorimeter, the temperature of the calorimeter increases by 10.0°C. The heat capacity of the calorimeter is 6.97 kJ/°C. Determine the energy flow, q (reaction).
(Multiple Choice)
4.8/5  (45)
(45)
When solid sodium hydroxide (NaOH) pellets are dissolved in water, the temperature of the water and beaker rises. This is an example of ________
(Multiple Choice)
4.8/5  (37)
(37)
Which statement A-D about the first law of thermodynamics ( Esystem = q + w) is not correct?
(Multiple Choice)
4.7/5  (38)
(38)
During a(n) ________ process, energy is transferred from the system to the surroundings.
(Multiple Choice)
4.7/5  (42)
(42)
Which of the following hydrocarbons has the greatest fuel value?
(Multiple Choice)
4.8/5  (42)
(42)
You hold a 50 g sphere of copper in one hand and a 25 g sphere of aluminum in the other hand. If both absorb energy at the same rate, which will come to your body temperature first and why? The specific heat capacities are 0.4 J/(g .°C) for copper and 0.9 J/(g .°C) for aluminum.
(Multiple Choice)
4.8/5  (28)
(28)
If a chemical reaction causes the temperature of the container to drop, it is a(n) ________ reaction.
(Multiple Choice)
4.7/5  (39)
(39)
Which statement below regarding the various heat capacities is not correct?
(Multiple Choice)
4.8/5  (39)
(39)
Showing 21 - 40 of 132
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)