Exam 5: Thermochemistry: Energy Changes in Reactions
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Energy that an object has by virtue of its motion is called ________
(Multiple Choice)
4.9/5  (41)
(41)
A 300 kg black bear hibernates in the winter. During hibernation the bear's body temperature drops from 37 to 31°C. How many grams of glucose must the bear metabolize to restore its body temperature to normal? Assume the bear's body is mostly water with a specific heat capacity of 4.2 J/(g . °C) and that all the energy from the combustion of glucose is used to raise the bear's body temperature. (Glucose: H(combustion) = -2,830 kJ/mol, molar mass = 180 g/mol)
(Multiple Choice)
4.8/5  (44)
(44)
The basal metabolic rate (BMR) is the minimum energy intake required to maintain a body that is awake and at rest. Although the rate varies with age, gender, health, and body mass, a reasonable value is 1,600 Cal per day. Walking can require 2.5 times the BMR, and exertion in work or sports can require 7 times the BMR. Using this information, calculate the energy expended by someone playing basketball, and determine how many minutes she could play with the energy from one jelly doughnut that has a mass of 50 g and a food value of 5 Cal/g.
(Essay)
5.0/5  (42)
(42)
The heating curve for a substance is shown below. The substance initially is a solid. It then becomes a liquid and a gas. Which of the line segments (I-V) represents the solid to liquid phase transition? 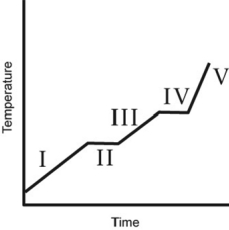
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following substances will release the most energy during combustion in air?
(Multiple Choice)
4.8/5  (38)
(38)
C8H18 (114 g/mol, d = 0.69 g/mL) is a good model for gasoline, and C16H34 (226 g/mol, d = 0.85 g/mL) is a good model for diesel fuel. These compounds have enthalpies of combustion of 5,460 kJ/mol and 10,800 kJ/mol, respectively. Calculate the fuel density (kJ/mL) of these compounds, and determine how many times more valuable diesel fuel is in terms of its energy content as measured by fuel density.
(Essay)
5.0/5  (33)
(33)
The cooling system in an automobile holds 10.0 L of ethylene glycol antifreeze. How much energy is absorbed when the temperature of the ethylene glycol goes from 20°C to 100°C? The density and specific heat capacity of ethylene glycol are 1.11 g/mL and 2.42 J/(g . °C), respectively.
(Multiple Choice)
4.8/5  (27)
(27)
Which statement A-D about the first law of thermodynamics ( Esystem = q + w) is not correct?
(Multiple Choice)
4.8/5  (34)
(34)
Suppose you eat a one-third pound hamburger without bread, cheese, or other items. Approximately how long would you need to walk to use up the calories in the hamburger? Assume the mass of the hamburger is 150 g with 3.6 Cal/g and that walking requires 2.5 times a basal metabolic rate of 280 kJ/hr.
(Multiple Choice)
4.9/5  (42)
(42)
In addition to identifying the reactants and products, a thermochemical reaction equation provides ________
(Multiple Choice)
4.9/5  (29)
(29)
In an experiment, 74.3 g of metallic copper was heated to 100.0°C and then quickly dropped into 200.0 mL of water in a calorimeter. The heat capacity of the calorimeter with the water was
875 J/°C. The initial temperature of the calorimeter was 27.5°C, and the final temperature after addition of the metal was 29.8°C. What is the value of the molar heat capacity of copper?
(Multiple Choice)
4.8/5  (44)
(44)
Benzoic acid is used to determine the heat capacity of bomb calorimeters because it can be obtained in pure form and its energy of combustion is known very accurately (-26.43 kJ/g). Determine the heat capacity of a calorimeter that had a temperature increase of 9.199°C when 3.500 g of benzoic acid was used.
(Multiple Choice)
4.8/5  (46)
(46)
Determine the standard enthalpy of formation for NO given the following information about the formation of NO2 under standard conditions, and H (NO2) = + 33.2 kJ/mol.
2NO(g) + O2(g) 2NO2(g) Hrxn = -114.2 kJ
(Multiple Choice)
4.8/5  (42)
(42)
Using the following data for water, determine the energy required to melt 1.00 g of ice (solid water) at its melting point. Boiling point 373 Melting point 273 Enthalpy of vaporization 2,260/ Enthalpy of fusion 334/ Specific heat capacity (solid) 2.11/(\cdot) Specific heat capacity (liquid) 4.18/(\cdot) Specific heat capacity (gas) 2.08/(\cdot)
(Multiple Choice)
4.9/5  (33)
(33)
Using the following data for water, determine the final temperature when 100 g of ice at -10°C is heated with 100 kJ of energy. Boiling point 373 Melting point 273 Enthalpy of vaporization 2,260/ Enthalpy of fusion 334/ Specific heat capacity (solid) 2.11/(\cdot) Specific heat capacity (liquid) 4.18/(\cdot) Specific heat capacity (gas) 2.08/(\cdot)
(Multiple Choice)
4.8/5  (39)
(39)
Work is done when a force moves an object some distance. Newton defined force as mass times acceleration. Acceleration is the rate at which velocity changes. What are the units of work?
(Multiple Choice)
4.9/5  (43)
(43)
Determine the change in enthalpy for the following reaction from the enthalpies of formation for the reactants and products.
(C2H2Cl2, +4.27 kJ/mol; C2H2Cl4, -155.6 kJ/mol)
Cl2(g) + C2H2Cl2(g) C2H2Cl4(g)
(Multiple Choice)
4.9/5  (49)
(49)
Which statement A-D about the relationship between energy and work is not correct?
(Multiple Choice)
4.8/5  (34)
(34)
Showing 101 - 120 of 132
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)