Exam 3: Stoichiometry: Mass, Formulas, and Reactions
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
List three reasons why the actual yield for a chemical reaction may differ from the theoretical yield.
(Essay)
4.9/5  (40)
(40)
A restaurant owner went to clean his oven using a solution that contained 150 g of sodium hydroxide (NaOH, 40.0 g/mol). He decided to neutralize the excess solution with an amount of vinegar containing 300 g of acetic acid (CH3COOH, 60.1 g/mol). The products are sodium acetate (82.0 g/mol) and water (18.0 g/mol). Identify the excess reactant and the amount that remains unreacted. The neutralization reaction equation is
NaOH(aq) + CH3COOH(aq) NaOOCCH3(aq) + H2O(l)
(Multiple Choice)
4.8/5  (36)
(36)
In one analysis, 3.01 *1013 molecules of ozone were found in 1.00 mL of an air sample. How many moles of ozone is this?
(Multiple Choice)
4.9/5  (44)
(44)
What are the stoichiometric coefficients for oxygen and water, respectively, in the balanced chemical reaction equation representing the combustion of butane (C4H10)?
(Multiple Choice)
4.8/5  (36)
(36)
A hydrocarbon molecule contains carbon and hydrogen atoms in equal numbers. Its molar mass is 130.18 g/mol. What is the molecular formula for the hydrocarbon?
(Multiple Choice)
4.8/5  (33)
(33)
A quart of water has a mass of 0.948 kg. How many moles of water (18.02 g/mol) is this?
(Multiple Choice)
4.8/5  (33)
(33)
Write the balanced reaction equation for the decomposition of ammonium nitrate into nitrogen gas, oxygen gas, and water vapor.
(Essay)
4.8/5  (31)
(31)
One form of elemental sulfur is a ring of eight sulfur atoms. How many moles of molecular oxygen are consumed when one mole of this allotrope burns to make sulfur trioxide?
(Multiple Choice)
5.0/5  (43)
(43)
Calcium carbide, CaC2, reacts with water to produce acetylene gas, C2H2, which is used in welding applications. The other reaction product is calcium hydroxide, Ca(OH)2. How much calcium carbide is needed to produce 26 kg of acetylene, assuming the reaction yield is 100%?
(Short Answer)
4.7/5  (34)
(34)
Water can be separated into its elements according to the following equation by electrolysis. If 20.0 g of water is decomposed by this method, how much oxygen gas is produced?
2H2O (l) 2H2(g) + O2(g)
(Multiple Choice)
4.9/5  (26)
(26)
Elemental analysis of the soot produced by a candle flame shows that it is 7.75% H and 92.3% C by mass. What is the empirical formula of this hydrocarbon?
(Multiple Choice)
4.9/5  (38)
(38)
What mass of phosphoric acid (H3PO4, 98.0 g/mol) is produced from the reaction of 10.0 g of P4O10 (284 g/mol) with excess water?
(Multiple Choice)
4.7/5  (30)
(30)
For which of the following compounds are the empirical and molecular formulas the same?
Acetic acid, found in vinegar, CH3COOH
Formaldehyde, used to preserve biological specimens, CH2O
Ethanol, found in beer and wine, CH3CH2OH
(Short Answer)
5.0/5  (31)
(31)
Which statement about a balanced chemical reaction equation is always correct?
(Multiple Choice)
4.7/5  (32)
(32)
One form of asbestos called chrysotile is considered to be a human carcinogen. Mass analysis reveals that it has the empirical formula Mg3Si2H4O9. If the molar mass is 554 g/mol, which molecular formula is correct?
(Multiple Choice)
4.9/5  (42)
(42)
Which molecules shown below have the same mass percent composition? 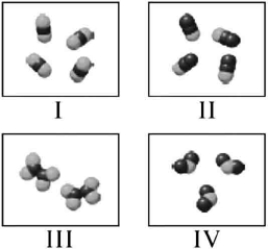
(Multiple Choice)
4.8/5  (29)
(29)
Household bleach contains 5.0% sodium hypochlorite (NaOCl) by mass. Household ammonia contains 5.0% NH3 by mass. If 50.00 g of bleach are mixed with 50.00 g of 5.0% ammonia, what mass of the toxic chloramine gas would be produced by the following reaction?
NaOCl + NH3 NH2Cl + NaOH
(Multiple Choice)
4.9/5  (36)
(36)
A gallon of water has a mass of 3.79 kg. How many moles of water (18.02 g/mol) is this?
(Multiple Choice)
4.9/5  (38)
(38)
An intermetallic titanium compound is used for medical implants and high quality jewelry. The composition of this compound is 70.68% titanium, 19.92% aluminum, and 9.400% vanadium. The molar masses of these elements are 47.88, 26.98, and 50.94 g/mol, respectively. What is the empirical formula of this compound?
(Multiple Choice)
4.9/5  (48)
(48)
A mass of 11.60 g of phosphoric acid was produced from the reaction of 10.00 g of P4O10 with 12.00 g water. What was the percent yield for this reaction?
(Multiple Choice)
4.8/5  (37)
(37)
Showing 21 - 40 of 133
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)