Exam 3: Stoichiometry: Mass, Formulas, and Reactions
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Which statement, A-D, regarding the carbon cycle is not correct?
(Multiple Choice)
4.9/5  (38)
(38)
Glucose (C6H12O6) is oxidized by molecular oxygen to carbon dioxide and water. How many O2 molecules are needed for each molecule of glucose that is oxidized?
(Multiple Choice)
5.0/5  (29)
(29)
The mass of 0.25 mol of some element is 8.0 g. What is the element?
(Multiple Choice)
4.9/5  (38)
(38)
How many mL of C16H34 are needed to react with 0.050 mol O2, assuming complete combustion occurs? The density of C16H34 is 0.80 g/mL.
(Multiple Choice)
4.8/5  (38)
(38)
How many oxygen atoms are there in 27.0 g of sodium sulfate (Na2SO4)?
(Multiple Choice)
4.9/5  (47)
(47)
Chemical analysis of an organic compound found the following composition: 40.0% C, 53.5% O, and 6.7% H. If the molar mass is 180.2 g/mol, how many empirical formula units are there in the molecular formula?
(Multiple Choice)
4.9/5  (33)
(33)
The average adult exhales about 1.0 kg of carbon dioxide each day. How much oxygen is needed in metabolizing glucose (C6H12O6, 180 g/mol) to make that much carbon dioxide?
(Multiple Choice)
4.8/5  (39)
(39)
Identify the list below that has these oxides arranged in order of increasing molar mass. Save time by looking at the periodic table and thinking, without actually doing any calculations.
(Multiple Choice)
4.8/5  (40)
(40)
Fe2O3(s) and powdered aluminum can react with great output of heat to form molten iron and Al2O3. When this reaction equation is balanced, what are the stoichiometric coefficients in the following order: Fe2O3, Al, Fe, Al2O3?
(Multiple Choice)
4.8/5  (35)
(35)
One form of asbestos called chrysotile is considered to be a human carcinogen. Mass analysis shows that the composition of chrysotile is 26.3% Mg, 20.2% Si, 1.45% H, and the remainder of the mass is oxygen. Determine the empirical formula of chrysotile.
(Multiple Choice)
4.8/5  (28)
(28)
Hydrogen peroxide decomposes to produce water and oxygen. Which relationship regarding the quantities of reactants and products associated with this reaction is not correct?
2H2O2 2H2O + O2
(Multiple Choice)
4.9/5  (42)
(42)
Ammonia, NH3, is an important industrial chemical. It is produced using the Haber process from nitrogen, N2, and hydrogen, H2. How much ammonia can be produced from 28 kg of nitrogen and 2 kg of hydrogen, assuming the reaction yield is 90%?
(Multiple Choice)
4.9/5  (36)
(36)
A balanced chemical reaction equation is to a reactant as __________
(Multiple Choice)
4.9/5  (36)
(36)
Write the balanced reaction equation for the combustion of isooctane (C8H18), which is found in gasoline.
(Essay)
4.9/5  (51)
(51)
Calcium hydride (CaH2) is so reactive with water that it can be used to remove traces of water from solvents other than water. If 24.6 g of calcium hydride is added to a large volume of solvent that contains 14.0 g of water, which of these remains, water or calcium hydride, after the reaction is complete? The reaction is:
CaH2(s) + 2H2O(l) Ca(OH)2(s) + 2H2(g)
(Multiple Choice)
4.9/5  (38)
(38)
Caffeine has an elemental analysis of 49.48% carbon, 5.190% hydrogen, 16.47% oxygen, and 28.85% nitrogen. It has a molar mass of 194.19 g/mol. What is the molecular formula of caffeine?
(Multiple Choice)
4.8/5  (37)
(37)
Which molecules shown below have the same empirical formula? 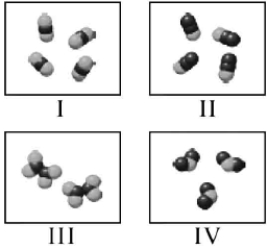
(Multiple Choice)
4.9/5  (36)
(36)
Write a definition of the phrase "stoichiometry of a chemical reaction."
(Essay)
4.8/5  (30)
(30)
Which statement about the following chemical reaction is not correct?
3H2 + N2 2NH3
(Multiple Choice)
4.7/5  (39)
(39)
The combustion of ethanol (CH3CH2OH, 46.1 g/mol) results in the formation of water and carbon dioxide. How many grams of carbon dioxide are produced when 46.1 g of ethanol burns?
(Multiple Choice)
4.8/5  (41)
(41)
Showing 41 - 60 of 133
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)