Exam 1: Measuring Matter and Energy
Exam 1: Measuring Matter and Energy99 Questions
Exam 2: Atomic Structure and Nuclear Radiation135 Questions
Exam 3: Compounds and Molecules164 Questions
Exam 4: Chemical Quantities and Chemical Reactions135 Questions
Exam 5: Changes of State and the Gas Laws85 Questions
Exam 6: Organic Chemistry: Hydrocarbons95 Questions
Exam 7: Organic Chemistry and Biomolecules101 Questions
Exam 8: Solutions, Colloids and Membranes93 Questions
Exam 9: Acids and Bases112 Questions
Exam 10: The Reactions of Organic Functional Groups in Biochemistry87 Questions
Exam 11: Carbohydrates: Structure and Function78 Questions
Exam 12: Lipids: Structure and Function108 Questions
Exam 13: Proteins: Structure and Function122 Questions
Exam 14: Nucleotides and Nucleic Acids107 Questions
Exam 15: Energy and Metabolism117 Questions
Select questions type
A medium apple provides about 80 Calories. How many calories are provided by the apple?
(Multiple Choice)
4.8/5  (37)
(37)
A child's height is 36.2 inches. What is her height in centimeters?
(Multiple Choice)
4.7/5  (32)
(32)
You have a 25-g sample of a metal and you would like to identify it. You are certain that the metal is either copper (specific heat = 0.093 cal/gC), lead (specific heat = 0.031 cal/gC), or aluminum (specific heat = 0.22 cal/gC). You run an experiment in which you find that the metal absorbs 6.2 calories of heat when it increases in temperature from 25C to 33C. Which metal is it?
(Multiple Choice)
5.0/5  (35)
(35)
Which of the following conversion factors are useful when converting 312 mg to kilograms? 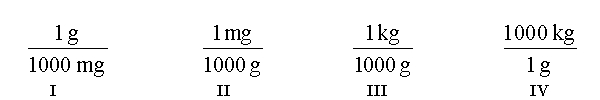
(Multiple Choice)
4.8/5  (39)
(39)
An aluminum ball is dropped into the graduated cylinder and the water level increases. If the ball has a volume of 6.8 mL, what is the new volume reading in the graduated cylinder?
(Multiple Choice)
4.9/5  (29)
(29)
A patient is given 5.00 mL of a medication that contains 0.0012 g of active ingredient per mL. To determine the amount of active ingredient administered, the product of the two numbers is calculated (5.00 mL 0.0012 g/mL). Using significant figures, what is this product?
(Multiple Choice)
5.0/5  (38)
(38)
There are five different objects with the diameters shown below. Which of these objects cannot be seen with the naked eye?
(Multiple Choice)
4.9/5  (39)
(39)
The smallest bone on the body, the stirrup-shaped stapes found in the middle ear, has a typical length of less than 0.33 cm. How long is typical maximum length of the stapes in inches?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following statements best describes specific heat?
(Multiple Choice)
4.9/5  (43)
(43)
What is the density of substance with a mass of 10.6 g and a volume of 12.0 mL?
(Multiple Choice)
4.9/5  (37)
(37)
You are asked to administer 3.50 mL of a liquid medication. Which measuring device would be the best choice for measuring 3.50 mL?
(Multiple Choice)
4.8/5  (27)
(27)
Tylenol is ordered for a child weighing 42 pounds at a dosage of 15 mg per kilogram of body weight. You need to determine how many mg of Tylenol should be administered to this child in a single dose. In order to answer this question, a conversion is used that is actually written within the body of the question. Which factor is this?
(Multiple Choice)
4.8/5  (38)
(38)
In which temperature scale(s) does zero mean that all molecular motion has stopped?
(Multiple Choice)
4.9/5  (44)
(44)
Consider a warm summer's day at the beach. While the sand feels warm on your feet, the water feels cool. Which statement is the best explanation for this phenomenon?
(Multiple Choice)
4.8/5  (43)
(43)
Showing 21 - 40 of 99
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)