Exam 1: Measuring Matter and Energy
Exam 1: Measuring Matter and Energy99 Questions
Exam 2: Atomic Structure and Nuclear Radiation135 Questions
Exam 3: Compounds and Molecules164 Questions
Exam 4: Chemical Quantities and Chemical Reactions135 Questions
Exam 5: Changes of State and the Gas Laws85 Questions
Exam 6: Organic Chemistry: Hydrocarbons95 Questions
Exam 7: Organic Chemistry and Biomolecules101 Questions
Exam 8: Solutions, Colloids and Membranes93 Questions
Exam 9: Acids and Bases112 Questions
Exam 10: The Reactions of Organic Functional Groups in Biochemistry87 Questions
Exam 11: Carbohydrates: Structure and Function78 Questions
Exam 12: Lipids: Structure and Function108 Questions
Exam 13: Proteins: Structure and Function122 Questions
Exam 14: Nucleotides and Nucleic Acids107 Questions
Exam 15: Energy and Metabolism117 Questions
Select questions type
Below are five descriptions of the kinetic and potential energy of objects. Which is a description of kinetic energy?
I. water moving a waterwheel
II. a skateboarder at the top of a halfpipe
III. the blades of a fan turning
IV. hot water molecules moving rapidly in a cup of tea
V. a parachutist ready to jump out of a plane
(Multiple Choice)
4.8/5  (37)
(37)
The number of significant figures in the measurement of 0.004500 cm3 is
(Multiple Choice)
4.8/5  (35)
(35)
Using significant figures, what is the product of 0.021 0.118 1020?
(Multiple Choice)
5.0/5  (37)
(37)
Water has a density of 1.0 g/mL. What is the mass of 25 mL of water?
(Multiple Choice)
4.7/5  (37)
(37)
This state of matter changes shape depending upon the shape of its container.
(Multiple Choice)
4.8/5  (44)
(44)
Which statement comparing the accuracies and precisions of ruler A and ruler B is correct? 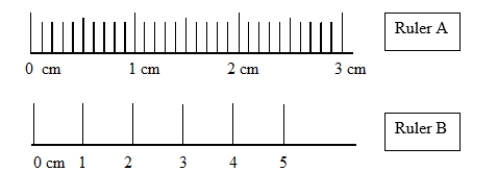
(Multiple Choice)
4.7/5  (31)
(31)
If you dropped a 6.0 g piece of aluminum (density = 2.70 g/mL) into a graduated cylinder containing 93.8 mL of water, what measurement would you read on the graduated cylinder?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following units of energy is equal to one thousand calories?
(Multiple Choice)
4.9/5  (30)
(30)
To make this table, four different people took three measurements each of the distance between the chemistry building and the cafeteria on campus. Each person used a different measuring device and therefore arrived at a different set of measurements. The true distance is 152 meters. Which person is the least precise and the least accurate? 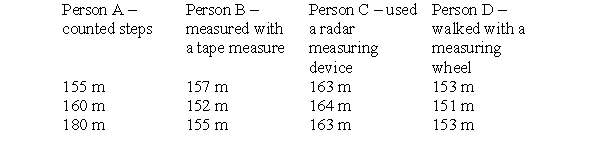
(Multiple Choice)
4.8/5  (35)
(35)
To make this table, four different people took three measurements each of the distance between the chemistry building and the cafeteria on campus. Each person used a different measuring device and therefore arrived at a different set of measurements. The true distance is 152 meters. Which person is precise, but not very accurate? 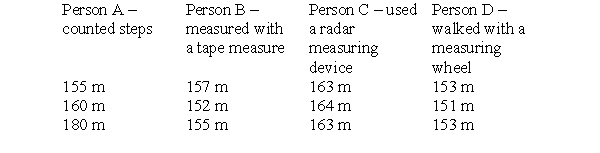
(Multiple Choice)
4.9/5  (31)
(31)
Which of the following measurements represents the least mass?
(Multiple Choice)
4.7/5  (39)
(39)
Which temperature scale(s) is/are relative (i.e., based on the freezing and boiling point of water)?
(Multiple Choice)
4.9/5  (36)
(36)
A patient has a kidney infection. Which of the following is most likely to be the specific gravity of the patient's urine?
(Multiple Choice)
4.8/5  (34)
(34)
How does the kinetic energy of the hot and cold bricks below change as time passes? 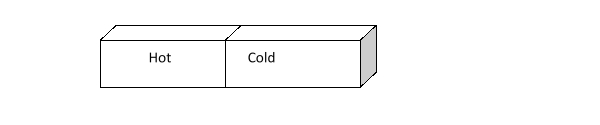
(Multiple Choice)
4.7/5  (31)
(31)
A patient's urine has a density of 1.010 g/mL. What is the specific gravity of the patient's urine?
(Multiple Choice)
4.8/5  (42)
(42)
Showing 81 - 99 of 99
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)