Exam 23: The Transition Elements and Their Coordination Compounds
Exam 1: Keys to the Study of Chemistry68 Questions
Exam 2: The Components of Matter104 Questions
Exam 3: Stoichiometry of Formulas and Equations96 Questions
Exam 4: Three Major Classes of Chemical Reactions105 Questions
Exam 5: Gases and the Kinetic-Molecular Theory103 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change79 Questions
Exam 7: Quantum Theory and Atomic Structure74 Questions
Exam 8: Electron Configuration and Chemical Periodicity81 Questions
Exam 9: Models of Chemical Bonding73 Questions
Exam 10: The Shapes of Molecules108 Questions
Exam 11: Theories of Covalent Bonding56 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes97 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids98 Questions
Exam 14: Periodic Patterns in the Main-Group Elements111 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon113 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions89 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions102 Questions
Exam 18: Acid-Base Equilibria106 Questions
Exam 19: Ionic Equilibria in Aqueous Systems115 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions85 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry56 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications90 Questions
Select questions type
A) State the requirement for two molecules to be optical isomers.
B) A complex ion MABCD2+ (where A, B, C and D are different unidentate ligands) rotates the plane of polarized light. Deduce the geometry of the complex and draw the optical isomers of this ionic formula.
(Essay)
4.9/5  (35)
(35)
A certain transition metal complex has the formula MX42+. If the metal ion has a d8 electron configuration,what is the shape of the complex?
(Multiple Choice)
4.8/5  (39)
(39)
A certain transition element has the stable oxidation states of +2, +3, +4, +5, and +6. In which state will the element be most likely to form a covalent bond with chlorine?
(Multiple Choice)
4.8/5  (39)
(39)
A) How many unpaired 3d electrons will there be in (i) high and (ii) low-spin complexes of Co(II)?
B) How can high and low-spin complexes be recognized and distinguished experimentally?
(Essay)
4.9/5  (45)
(45)
Which of the following ligands could participate in linkage isomerism?
(Multiple Choice)
4.8/5  (34)
(34)
Iron(III) forms an octahedral complex with the ligand CN¯. How many unpaired electrons are in the d orbitals of iron?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following ligands is most likely to form a high spin octahedral complex with cobalt(II)?
(Multiple Choice)
4.8/5  (44)
(44)
Which of the following transition elements can achieve the largest oxidation number?
(Multiple Choice)
4.8/5  (33)
(33)
The dxy and the 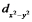 orbitals both lie in the xy plane, yet for a metal ion in an octahedral complex the energy of the dxy orbital is lower than that of the
orbitals both lie in the xy plane, yet for a metal ion in an octahedral complex the energy of the dxy orbital is lower than that of the 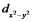 orbital. Explain this using the arguments of crystal field theory.
orbital. Explain this using the arguments of crystal field theory.
(Essay)
4.8/5  (38)
(38)
Which of the following elements has the ground state electron configuration, [Xe]4f145d106s1?
(Multiple Choice)
4.8/5  (38)
(38)
Of the 3d transition series of elements, zinc has the greatest atomic radius.
(True/False)
4.8/5  (42)
(42)
If a solution absorbs green light, what is its likely color?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 81 - 92 of 92
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)