Exam 23: The Transition Elements and Their Coordination Compounds
Exam 1: Keys to the Study of Chemistry68 Questions
Exam 2: The Components of Matter104 Questions
Exam 3: Stoichiometry of Formulas and Equations96 Questions
Exam 4: Three Major Classes of Chemical Reactions105 Questions
Exam 5: Gases and the Kinetic-Molecular Theory103 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change79 Questions
Exam 7: Quantum Theory and Atomic Structure74 Questions
Exam 8: Electron Configuration and Chemical Periodicity81 Questions
Exam 9: Models of Chemical Bonding73 Questions
Exam 10: The Shapes of Molecules108 Questions
Exam 11: Theories of Covalent Bonding56 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes97 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids98 Questions
Exam 14: Periodic Patterns in the Main-Group Elements111 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon113 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions89 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions102 Questions
Exam 18: Acid-Base Equilibria106 Questions
Exam 19: Ionic Equilibria in Aqueous Systems115 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions85 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry56 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications90 Questions
Select questions type
In the formation of a transition metal complex, the central metal atom or ion acts as
(Multiple Choice)
4.8/5  (29)
(29)
Consider the following octahedral complex structures, each involving ethylene diamine and two different, unidentate ligands X and Y. 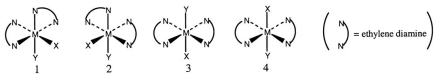 Which one, if any, of the following is a pair of optical isomers?
Which one, if any, of the following is a pair of optical isomers?
(Multiple Choice)
4.9/5  (43)
(43)
Of the 3d transition series of elements, scandium has the greatest atomic radius.
(True/False)
4.7/5  (29)
(29)
What is the highest possible oxidation state for molybdenum, Mo?
(Multiple Choice)
4.7/5  (38)
(38)
Mercury(II) compounds are assimilated into the food chain because
(Multiple Choice)
4.8/5  (36)
(36)
According to Valence Bond theory, in the square planar Ni(CN)42¯ complex ion, the orbital hybridization pattern is:
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following ions is most likely to form colored compounds?
(Multiple Choice)
4.8/5  (27)
(27)
The most common oxidation state for ions of the transition elements is:
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following octahedral complexes should have the largest crystal field splitting energy, ?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following ions could exist in only the high-spin state in an octahedral complex?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following should be the strongest reducing agent?
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following will be the strongest oxidizing agent?
(Multiple Choice)
5.0/5  (40)
(40)
Which of the following ions is least likely to form colored compounds?
(Multiple Choice)
4.9/5  (31)
(31)
All atoms of the first transition series of elements have the ground state electronic configuration [Ar]4s23dx, where x is an integer from 1 to 10.
(True/False)
4.8/5  (42)
(42)
In the spectrochemical series, which one of the following ligands has the strongest field?
(Multiple Choice)
4.9/5  (41)
(41)
Showing 21 - 40 of 92
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)