Exam 23: The Transition Elements and Their Coordination Compounds
Exam 1: Keys to the Study of Chemistry68 Questions
Exam 2: The Components of Matter104 Questions
Exam 3: Stoichiometry of Formulas and Equations96 Questions
Exam 4: Three Major Classes of Chemical Reactions105 Questions
Exam 5: Gases and the Kinetic-Molecular Theory103 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change79 Questions
Exam 7: Quantum Theory and Atomic Structure74 Questions
Exam 8: Electron Configuration and Chemical Periodicity81 Questions
Exam 9: Models of Chemical Bonding73 Questions
Exam 10: The Shapes of Molecules108 Questions
Exam 11: Theories of Covalent Bonding56 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes97 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids98 Questions
Exam 14: Periodic Patterns in the Main-Group Elements111 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon113 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions89 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions102 Questions
Exam 18: Acid-Base Equilibria106 Questions
Exam 19: Ionic Equilibria in Aqueous Systems115 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions85 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry56 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications90 Questions
Select questions type
If M represents a transition element, which of the following oxides should be the least basic?
(Multiple Choice)
4.8/5  (34)
(34)
According to valence bond theory, what would be the set of hybrid orbitals used when a Period 4 transition metal forms a square planar complex?
(Multiple Choice)
4.8/5  (42)
(42)
What is the difference between a coordination compound and a complex ion?
(Essay)
4.8/5  (45)
(45)
A) Explain how the crystal field theory can use the magnitude of the splitting energy to provide an explanation of the color and magnetic properties of octahedral complexes.
B) In promoting an electron from the t2g set of orbitals to the eg set, an octahedral complex absorbs a photon with a wavelength of 523 nm. Calculate the value of in the complex, in kJ/mol.
(Essay)
4.9/5  (31)
(31)
According to valence bond theory, what would be the set of hybrid orbitals used when a Period 4 transition metal forms a tetrahedral complex?
(Multiple Choice)
4.8/5  (40)
(40)
What geometry is particularly common for complexes of d10 metal ions?
(Short Answer)
4.8/5  (32)
(32)
Which one of the following normally acts as a bidentate ligand in complexes with transition metal ions?
(Multiple Choice)
4.9/5  (33)
(33)
What is the coordination number of cobalt in the complex ion [Co(en)Cl4]¯? (en = ethylenediamine)
(Multiple Choice)
4.8/5  (35)
(35)
In a coordination compound involving a complex ion of square planar geometry, which of the following types of isomerism is/are never possible?
(Multiple Choice)
4.8/5  (39)
(39)
The ground state electron configuration of a transition element atom cannot have more than one incomplete subshell.
(True/False)
4.8/5  (46)
(46)
The M2+ ions of the first transition series of elements all have the general electronic configuration [Ar]4s23dx, where x is an integer from 1 to 8.
(True/False)
4.9/5  (29)
(29)
Which of the following is not a property of silver that is important in black and white photography?
(Multiple Choice)
4.8/5  (36)
(36)
In the compound [Ni(en)2(H2O)2]SO4 (where en = ethylenediamine) the oxidation number and coordination number of nickel are, respectively:
(Multiple Choice)
4.9/5  (33)
(33)
A feature of transition metal chemistry is that these elements exhibit multiple oxidation states. Which one of the following elements exhibits the smallest number of different oxidation states?
(Multiple Choice)
4.9/5  (41)
(41)
Consider the following octahedral complex structures, each involving ethylene diamine and two different, unidentate ligands X and Y. 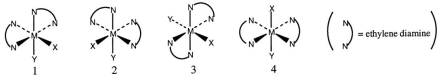 Which one of the following statements about these structures is incorrect?
Which one of the following statements about these structures is incorrect?
(Multiple Choice)
4.8/5  (35)
(35)
Showing 41 - 60 of 92
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)