Exam 2: Atoms, Ions, and Molecules the Building Blocks of Matter
Exam 1: Matter and Energy an Atomic Perspective138 Questions
Exam 2: Atoms, Ions, and Molecules the Building Blocks of Matter143 Questions
Exam 3: Atomic Structure Explaining the Properties of Elements175 Questions
Exam 4: Chemical Bonding Understanding Climate Change182 Questions
Exam 5: Bonding Theories Explaining Molecular Geometry141 Questions
Exam 6: Intermolecular Forces Attractions Between Particles87 Questions
Exam 7: Stoichiometry Mass Relationships and Chemical Reactions140 Questions
Exam 8: Aqueous Solutions Chemistry of the Hydrosphere180 Questions
Exam 9: Thermochemistry Energy Changes in Chemical Reactions215 Questions
Exam 10: Properties of Gases the Air We Breathe164 Questions
Exam 11: Properties of Solutions Their Concentrations and Colligative Properties130 Questions
Exam 12: Thermodynamics Why Chemical Reactions Happen130 Questions
Exam 13: Chemical Kinetics Clearing the Air172 Questions
Exam 14: Chemical Equilibrium Equal but Opposite Reaction Rates119 Questions
Exam 15: Acid-Base Equilibria Proton Transfer in Biological Systems123 Questions
Exam 16: Additional Aqueous Equilibria Chemistry and the Oceans114 Questions
Exam 17: Electrochemistry the Quest for Clean Energy135 Questions
Exam 18: The Solid State a Particulate View170 Questions
Exam 19: Organic Chemistry Fuels, Pharmaceuticals, and Modern Materials145 Questions
Exam 20: Biochemistry the Compounds of Life153 Questions
Exam 21: Nuclear Chemistry the Risks and Benefits168 Questions
Exam 22: The Main Group Elements Life and the Periodic Table116 Questions
Exam 23: Transition Metals Biological and Medical Applications119 Questions
Select questions type
Which of the following compounds contains the most nitrogen atoms per formula?
(Multiple Choice)
4.7/5  (44)
(44)
Silicon brass contains 82.0% Cu, 14.0% Zn, and 4.00% Si by mass, and its density is 8.28 g/cm3.How many moles of silicon are present in 22.0 cm3 of silicon brass?
(Multiple Choice)
4.8/5  (33)
(33)
Zinc oxide, a combination of zinc and oxygen, is found in many skin ointments.What formula best describes this compound?
(Multiple Choice)
4.7/5  (36)
(36)
TNT, or trinitrotoluene, has the chemical formula C7H5N3O6.How many grams of nitrogen are present in 25 g TNT (227.13 g/mol)?
(Multiple Choice)
4.7/5  (34)
(34)
If the nucleus of an atom has a radius of about 5 fm and a mass of about 2 *10-21 g, what is its approximate density? (The volume of a sphere = 4 r3/3.)
(Multiple Choice)
4.7/5  (41)
(41)
Gallium has two naturally occurring isotopes with the following masses and natural abundances.Calculate the average atomic mass of Ga.
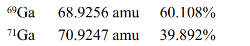
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following statements regarding the discovery of isotopes is FALSE?
(Multiple Choice)
4.9/5  (32)
(32)
How many bromine atoms are in a formula unit of calcium bromide?
(Short Answer)
4.9/5  (40)
(40)
What is the mass in (amu) of one molecule of glucose, C6H12O6?
(Short Answer)
4.9/5  (36)
(36)
Identify the element based on the following values for its three isotopes: 38.9637 amu (93.08%), 39.9640 amu (0.012%), and 40.9618 amu (6.91%).
(Multiple Choice)
4.8/5  (48)
(48)
Nuclei with certain numbers of protons and neutrons (or combinations thereof) appear to be more stable than others."Magic numbers" that are consistent with known nuclides are 2, 8, 20, 28, 50, 82, and 126, with 180 and 306 being hypothesized as the next in the series.Using this information, along with your knowledge of atoms and isotopes, you try to synthesize a new atom (symbol X) based on stable combinations of nucleons.Which do you think is a likely candidate?
(Multiple Choice)
4.8/5  (37)
(37)
Calculate the formula unit mass of sodium phosphate (Na3PO4) in which all of the phosphorus is 32P, a radioactive isotope of phosphorus used in medical applications.A32P atom has an atomic mass of
31.97 amu.
(Short Answer)
4.8/5  (32)
(32)
Showing 41 - 60 of 143
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)