Exam 15: The Laws of Thermodynamics
Exam 1: Introduction, Measurement, Estimating29 Questions
Exam 2: Describing Motion: Kinematics in One Dimension527 Questions
Exam 3: Kinematics in Two Dimensions; Vectors183 Questions
Exam 4: Dynamics: Newtons Laws of Motion146 Questions
Exam 5: Circular Motion; Gravitation105 Questions
Exam 6: Work and Energy153 Questions
Exam 7: Linear Momentum139 Questions
Exam 8: Rotational Motion148 Questions
Exam 9: Static Equilibrium; Elasticity and Fracture83 Questions
Exam 10: Fluids98 Questions
Exam 11: Oscillations and Waves114 Questions
Exam 12: Sound21 Questions
Exam 13: Temperature and Kinetic Theory87 Questions
Exam 14: Heat88 Questions
Exam 15: The Laws of Thermodynamics78 Questions
Exam 16: Electric Charge and Electric Field99 Questions
Exam 17: Electric Potential107 Questions
Exam 18: Electric Currents96 Questions
Exam 19: Dc Circuits384 Questions
Exam 20: Magnetism164 Questions
Exam 21: Electromagnetic Induction and Faradays Law60 Questions
Exam 22: Electromagnetic Waves167 Questions
Exam 23: Light: Geometric Optics144 Questions
Exam 24: The Wave Nature of Light58 Questions
Exam 25: Optical Instruments156 Questions
Exam 26: The Special Theory of Relativity126 Questions
Exam 27: Early Quantum Theory and Models of the Atom192 Questions
Exam 28: Quantum Mechanics of Atoms74 Questions
Exam 29: Molecules and Solids26 Questions
Exam 30: Nuclear Physics and Radioactivity153 Questions
Exam 31: Nuclear Energy; Effects and Uses of Radiation36 Questions
Exam 32: Elementary Particles19 Questions
Exam 33: Astrophysics and Cosmology25 Questions
Select questions type
A real (non-Carnot) heat engine, operating between heat reservoirs at temperatures of 450 and , performs of net work, and rejects of heat in a single cycle.
(a) What is the thermal efficiency of this heat engine?
(b) What is the maximum efficiency it could possibly have?
Free
(Short Answer)
4.8/5  (34)
(34)
Correct Answer:
(a) 0.29 (b) 0.40
A heat engine with an efficiency of performs of work. How much heat is discharged to the lower temperature reservoir?
Free
(Multiple Choice)
4.8/5  (27)
(27)
Correct Answer:
D
A container of ideal gas at STP undergoes an isothermal expansion and its entropy changes by . How much work does it do?
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
C
What is the efficiency of an ideal Carnot engine operating between a reservoir in which ice and water coexist, and a reservoir in which water and steam coexist? The pressure is constant at atm for both reservoirs.
(Multiple Choice)
4.8/5  (34)
(34)
A certain heat engine extracts of heat from a hot temperature reservoir and discharges of heat to a cold temperature reservoir. What is the efficiency of this engine?
(Multiple Choice)
4.8/5  (31)
(31)
One of the most efficient engines built so far has the following characteristics: The combustion chamber temperature is , the exhaust temperature cal of fuel produces of work in one hour. ( )
(a) What is the actual efficiency of this engine?
(b) What is the power output of this engine?
(c) What would be the maximum possible efficiency for an engine using the same temperature extremes?
(Short Answer)
4.8/5  (34)
(34)
An ideal gas undergoes the process shown in the diagram. In this figure, , and . How much work is done by the system in this process?
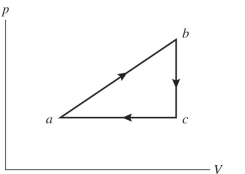
(Multiple Choice)
4.8/5  (36)
(36)
A certain gas is compressed adiabatically. The amount of work done on the gas is . What is the change in the internal (thermal) energy of the gas?
(Multiple Choice)
4.9/5  (41)
(41)
A gas expands from an initial volume of to a final volume of at a constant pressure of . How much work is done by the gas during this expansion?
(Multiple Choice)
4.8/5  (27)
(27)
A cyclic process is carried out on an ideal gas such that it returns to its initial state at the end of a cycle, as shown in the diagram in the figure. If the process is carried out in a clockwise sense around the enclosed area, as shown on the figure, then the magnitude of the enclosed area represents
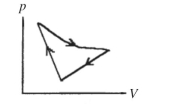
(Multiple Choice)
4.9/5  (28)
(28)
What is the change in entropy when of water at are turned into steam at ? The latent heat of vaporization of water is .
(Multiple Choice)
4.8/5  (40)
(40)
When of water at freezes, what is the change in entropy of the water? The latent heat of fusion of water is .
(Multiple Choice)
4.8/5  (20)
(20)
The ocean thermal energy conversion project uses the surface water near tropical islands with a temperature of as the hot temperature reservoir, and the water at some depth, with a temperature of , as the cold temperature reservoir for a heat engine. What is the maximum possible efficiency of an engine running between those two temperatures?
(Multiple Choice)
4.9/5  (39)
(39)
A monatomic ideal gas undergoes an isothermal expansion at , as the volume increased from to . The final pressure is . What is the change in the internal (thermal) energy of the gas during this process?
(Multiple Choice)
4.8/5  (36)
(36)
During each cycle, a refrigerator removes of heat from the freezing compartment and ejects into a room.
(a) How much work per cycle is required each cycle to run this refrigerator?
(b) What is the coefficient of performance of this refrigerator?
(Short Answer)
4.7/5  (37)
(37)
An ideal Carnot engine is operated between a hot and a cold reservoir. The temperature difference between the two reservoirs is . If the efficiency of this ideal engine is , find the temperature of the cold reservoir in degrees Celsius.
(Short Answer)
4.8/5  (36)
(36)
An inventor tries to sell you his new heat engine that takes in of heat at on each cycle, expels at , and does of work. Would it be wise to invest in this engine? Back up your conclusion with numerical calculations.
(Short Answer)
4.9/5  (31)
(31)
The figure shows a diagram for mol of ideal gas that undergoes the process . The gas then undergoes an isochoric heating from point 2 until the pressure is restored to the value it had at point 1. What is the final temperature of the gas? .
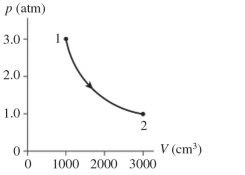
(Multiple Choice)
4.8/5  (36)
(36)
A coal-fired plant generates of electric power. The plant uses of coal each day, and the heat of combustion of coal is . The steam that drives the turbines is at a temperature of , and the exhaust water is at .
(a) What is the overall efficiency of the plant for generating electric power?
(b) How much thermal energy is exhausted each day?
(c) Using the same heat reservoirs, what is the maximum possible efficiency for a heat engine?
(Short Answer)
4.7/5  (35)
(35)
Showing 1 - 20 of 78
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)