Exam 17: Temperature, Thermal Expansion, and the Ideal Gas Law
Exam 1: Introduction, Measurement, Estimating71 Questions
Exam 2: Describing Motion: Kinematics in One Dimension119 Questions
Exam 3: Kinematics in Two or Three Dimensions; Vectors100 Questions
Exam 4: Dynamics: Newtons Laws of Motion86 Questions
Exam 5: Using Newtons Laws: Friction, Circular Motion, Drag Forces68 Questions
Exam 6: Gravitation and Newtons6 Synthesis64 Questions
Exam 7: Work and Energy69 Questions
Exam 8: Conservation of Energy95 Questions
Exam 9: Linear Momentum85 Questions
Exam 10: Rotational Motion99 Questions
Exam 11: Angular Momentum; General Rotation45 Questions
Exam 12: Static Equilibrium; Elasticity and Fracture61 Questions
Exam 13: Fluids112 Questions
Exam 14: Oscillations102 Questions
Exam 15: Wave Motion74 Questions
Exam 16: Sound75 Questions
Exam 17: Temperature, Thermal Expansion, and the Ideal Gas Law83 Questions
Exam 18: Kinetic Theory of Gases37 Questions
Exam 19: Heat and the First Law of Thermodynamics96 Questions
Exam 20: Second Law of Thermodynamics77 Questions
Exam 21: Electric Charge and Electric Field97 Questions
Exam 22: Gausss Law44 Questions
Exam 23: Electric Potential70 Questions
Exam 24: Capacitance, Dielectrics, Electric Energy Storage73 Questions
Exam 25: Electric Currents and Resistance71 Questions
Exam 26: Dc Circuits110 Questions
Exam 27: Magnetism102 Questions
Exam 28: Sources of Magnetic Field63 Questions
Exam 29: Electromagnetic Induction and Faradays Law116 Questions
Exam 30: Inductance, Electromagnetic Oscillations, and Ac Circuits108 Questions
Exam 31: Maxwells Equations and Electromagnetic Waves76 Questions
Exam 32: Light: Reflection and Refraction118 Questions
Exam 33: Lenses and Optical Instruments134 Questions
Exam 34: The Wave Nature of Light; Interference77 Questions
Exam 35: Diffraction and Polarization68 Questions
Exam 36: Special Theory of Relativity69 Questions
Exam 37: Early Quantum Theory and Models of the Atom95 Questions
Exam 38: Quantum Mechanics42 Questions
Exam 39: Quantum Mechanics of Atoms62 Questions
Exam 40: Molecules and Solids56 Questions
Exam 41: Nuclear Physics and Radioactivity82 Questions
Exam 42: Nuclear Energy: Efects and Uses of Radiation69 Questions
Exam 43: Elementary Particle66 Questions
Exam 44: Astrophysics and Cosmology36 Questions
Select questions type
On a hot summer day, you turn the thermostat in your house way down. Assume that your house is well sealed and that no air enters or leaves the house. When the air temperature in your house falls a short while later, what statement is correct regarding the air pressure?
(Multiple Choice)
4.9/5  (31)
(31)
A mole of diatomic oxygen molecules and a mole of diatomic nitrogen molecules at STP have
(Multiple Choice)
4.9/5  (44)
(44)
The Concorde's exterior is made of aluminum, which has a coefficient of linear expansion of 24 × 10-6K-1. At 15°C, the Concorde measures 62.1 m in length. When the plane is in flight, friction with the air increases the temperature of the exterior skin to 200°C. What is the change in the length of the outer skin of the Concorde?
(Multiple Choice)
4.8/5  (36)
(36)
An ideal gas occupies 400 L at an absolute pressure of 500 kPa. Find the absolute pressure if the volume changes to 950 L and the temperature remains constant.
(Multiple Choice)
4.7/5  (37)
(37)
If a thermometer measures the temperature of two objects as being equal, you can conclude that if the objects are placed in thermal contact, no heat will flow between them.
(True/False)
4.8/5  (37)
(37)
A ideal gas constant volume gas thermometer has pressure 2.00 atm at the triple point of water. What is the pressure when the temperature in the thermometer is 500°C?
(Multiple Choice)
4.9/5  (28)
(28)
The volume of a given amount of gas is inversely proportional to the absolute temperature when the pressure is kept constant.
(True/False)
4.8/5  (43)
(43)
A constant volume closed container of gas is at a pressure 1.00 × 105 N/m2 and a temperature 20°C. What is the pressure if the temperature of the gas is increased to 60.0°C?
(Multiple Choice)
4.8/5  (38)
(38)
How many moles of H2O molecules are in a 4.00 m3 container at a pressure 8.00 × 105 N/m2 and temperature 600°C?
(Multiple Choice)
4.9/5  (40)
(40)
How many water molecules are there in 36 g of water? Express your answer as a multiple of Avogadro's number NA. (The molecular structure of a water molecule is H2O.)
(Multiple Choice)
4.9/5  (33)
(33)
FIGURE 17-2 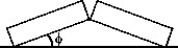 -Two identical concrete slabs lie flat and in contact with each other as shown in Fig. 17-2. If the temperature increases by 40°C, the lower edges opposite the contact edges remained fixed in position, and the lower edges of the contact side remain in contact, at what angle φ will the slabs be tilted? The coefficient of thermal expansion of the concrete is 10 × 10-6/K.
-Two identical concrete slabs lie flat and in contact with each other as shown in Fig. 17-2. If the temperature increases by 40°C, the lower edges opposite the contact edges remained fixed in position, and the lower edges of the contact side remain in contact, at what angle φ will the slabs be tilted? The coefficient of thermal expansion of the concrete is 10 × 10-6/K.
(Multiple Choice)
5.0/5  (38)
(38)
Equal volumes of gas at the same pressure and temperature contain equal numbers of molecules.
(True/False)
4.8/5  (30)
(30)
A bimetallic strip is made by bonding together a 1.0-mm thick and 15.0-cm long steel strip to a similar strip made of aluminum. The strip is straight when the temperature is 20.0°C. What will the radius of curvature of the strip be when the temperature is 40.0°C? The coefficient of thermal expansion of steel is 10.5 × 10-6K-1 and that of aluminum is 23.0 × 10-6K-1.
(Multiple Choice)
4.9/5  (32)
(32)
On a cold winter day, you turn up the thermostat in your house. Assume that your house is well sealed and that no air enters or leaves the house. When the air temperature in your house rises a short while later, what statement is correct regarding the air pressure?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 21 - 40 of 83
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)