Exam 4: Three Major Classes of Chemical Reactions
Exam 1: Keys to the Study of Chemistry: Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter101 Questions
Exam 3: Stoichiometry of Formulas and Equations72 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory98 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes95 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements103 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions98 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and Reaction Direction85 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work101 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds81 Questions
Exam 24: Nuclear Reactions and Their Applications82 Questions
Select questions type
Identify the oxidizing agent in the following redox reaction. Hg2+(aq) + Cu(s) → Cu2+(aq) + Hg(l)
(Multiple Choice)
4.9/5  (32)
(32)
Calcium chloride is used to melt ice and snow on roads and sidewalks and to remove water from organic liquids. Calculate the molarity of a solution prepared by diluting 165 mL of 0.688 M calcium chloride to 925.0 mL.
(Multiple Choice)
4.8/5  (42)
(42)
1.0M aqueous solutions of the following substances are prepared. Which one would you expect to have the lowest electrical conductivity?
(Multiple Choice)
4.9/5  (35)
(35)
Aqueous potassium iodate (KIO3) and potassium iodide (KI) react in the presence of dilute hydrochloric acid (HCl), as shown below. KIO3(aq) + 5KI(aq) + 6HCl(aq) → 3I2(aq) + 6KCl(aq) + 3H2O(l)
What mass of iodine (I2) is formed when 15.0 mL of 0.0050 M KIO3 solution reacts with 30.0 mL of 0.010 M KI solution in the presence of excess HCl?
(Multiple Choice)
4.9/5  (29)
(29)
During a titration, the following data were collected. A 25 mL portion of an unknown acid solution was titrated with 1.0 M NaOH. It required 65 mL of the base to neutralize the sample. How many moles of acid are present in 3.0 liters of this unknown solution?
(Multiple Choice)
4.8/5  (39)
(39)
How many moles of H+(aq) ions are present in 1.25 L of 0.75 M nitric acid?
(Multiple Choice)
4.9/5  (45)
(45)
All acid-base reactions produce a salt and water as the only products.
(True/False)
4.9/5  (36)
(36)
Sodium thiosulfate, Na2S2O3, is used as a "fixer" in black and white photography. Identify the reducing agent in the reaction of thiosulfate with iodine. 2S2O32-(aq) + I2(aq) → S4O62-(aq) + 2I-(aq)
(Multiple Choice)
4.9/5  (31)
(31)
Consider the reaction: 3Co2+(aq) + 6NO3(aq) + 6Na+(aq) + 2PO43-(aq) → Co3(PO4)2(s) + 6Na+(aq) + 6NO3(aq)
Identify the net ionic equation for this reaction.
(Multiple Choice)
4.9/5  (30)
(30)
Select the classification for the following reaction. 2NaCl(l) 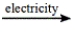 2Na(l) + Cl2(g)
2Na(l) + Cl2(g)
(Multiple Choice)
4.9/5  (33)
(33)
The oxidation numbers of P, S, and Cl in H2PO2-, H2S, and KClO4 are, respectively
(Multiple Choice)
4.9/5  (41)
(41)
Showing 101 - 111 of 111
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)