Exam 14: Oxidation-Reduction Reactions
Exam 1: Matter and Energy121 Questions
Exam 2: Atoms, Ions, and the Periodic Table143 Questions
Exam 3: Chemical Compounds113 Questions
Exam 4: Chemical Composition144 Questions
Exam 5: Chemical Reactions and Equations129 Questions
Exam 6: Quantities in Chemical Reactions133 Questions
Exam 7: Electron Structure of the Atom133 Questions
Exam 8: Chemical Bonding124 Questions
Exam 9: The Gaseous State121 Questions
Exam 10: The Liquid and Solid States118 Questions
Exam 11: Solutions119 Questions
Exam 12: Reaction Rates and Chemical Equilibrium110 Questions
Exam 13: Acids and Bases137 Questions
Exam 14: Oxidation-Reduction Reactions120 Questions
Exam 15: Nuclear Chemistry106 Questions
Exam 16: Organic Chemistry129 Questions
Exam 17: Biochemistry116 Questions
Select questions type
Given the following information about the activity series, predict whether the given reaction will occur or not. If it does occur, write a balanced equation. Cu < H2 < Sn < Fe < Zn < Mg Fe(s)+ SnCl2(aq)→
Free
(Multiple Choice)
4.9/5  (43)
(43)
Correct Answer:
C
The potential of a voltaic cell is greatest in cells constructed of strong oxidizing and reducing agents.
Free
(True/False)
4.7/5  (47)
(47)
Correct Answer:
True
Equations that are written to represent either an oxidation reaction or a reduction reaction are called half-reactions.
Free
(True/False)
4.9/5  (34)
(34)
Correct Answer:
True
Consider the following oxidation-reduction reaction: 2Fe3+(aq)+ 2Hg(l)+ 2Cl−(aq)→ 2Fe2+(aq)+ Hg2Cl2(s)Which one of the following pairs correctly indicates the oxidizing agent and the reducing agent in this reaction? Oxidizing agent Reducing agent
(Multiple Choice)
4.8/5  (38)
(38)
Given the following information about the activity series, predict whether the given reaction will occur or not. If it does occur, write a balanced equation. Cu < H2 < Sn < Fe < Zn < Mg Mg(s)+ ZnCl2(aq)→
(Multiple Choice)
4.9/5  (43)
(43)
The figure shows a molecular-level representation of the following voltaic cell: Mg(s)+ Sn2+(aq)→ Mg2+(aq)+ Sn(s)When drawing the "after" representation one would note that 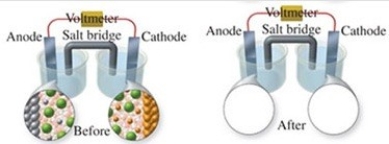
(Multiple Choice)
5.0/5  (37)
(37)
The figure shows the electrolysis of molten AlCl3. What reaction occurs at the anode of this cell? 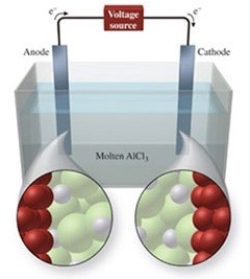
(Multiple Choice)
4.8/5  (37)
(37)
Consider the reaction: N2(g)+ 3H2(g)→ 2NH3(g)Which of the following statements is correct?
(Multiple Choice)
4.9/5  (41)
(41)
Consider a voltaic cell that corresponds to the following reaction: Zn(s)+ Cu2+(aq)→ Zn2+(aq)+ Cu(s)Which of the following statements is correct?
(Multiple Choice)
4.8/5  (36)
(36)
In which compound does phosphorus have an oxidation number of 3+?
(Multiple Choice)
4.8/5  (29)
(29)
An electrolytic cell is an electrochemical cell in which an oxidation-reduction reaction causes electrons to flow through an external circuit.
(True/False)
4.8/5  (38)
(38)
A mercury button battery that is used in watches and calculators is powered by the following reaction: Zn(s)+ HgO(s)→ ZnO(s)+ Hg(s)The substance that is oxidized in this battery is:
(Multiple Choice)
4.8/5  (42)
(42)
Given the following information about the activity series, predict whether the given reaction will occur or not. If it does occur, write a balanced equation. Cu < H2 < Sn < Fe < Zn < Mg
Mg(s)+ HCl(aq)→
(Multiple Choice)
5.0/5  (40)
(40)
The following reaction occurs in a lead storage battery: PbO2(s)+ Pb(s)+ H2SO4(aq)⇌ 2PbSO4(s)+ 2H2O(l)Which statement is true?
(Multiple Choice)
4.9/5  (45)
(45)
Consider the reaction: Ca(s)+ 2H2O(l)→ Ca(OH)2(aq)+ H2(g)Which of the following statements is correct?
(Multiple Choice)
4.8/5  (35)
(35)
Consider the reaction: Zn(s)+ H2SO4(aq)→ ZnSO4(aq)+ H2(g)Which species is oxidized, and how many electrons are transferred per atom of zinc that reacts?
(Multiple Choice)
4.9/5  (29)
(29)
The figure shows the electrolysis of molten NaI. What reaction occurs at the cathode of this cell? 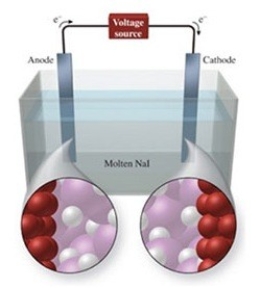
(Multiple Choice)
4.8/5  (29)
(29)
In a fuel cell that uses gaseous hydrogen and oxygen as reactants, which one of the following reactions is occurring at the anode?
(Multiple Choice)
4.7/5  (40)
(40)
Showing 1 - 20 of 120
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)