Exam 6: Quantities in Chemical Reactions
Exam 1: Matter and Energy121 Questions
Exam 2: Atoms, Ions, and the Periodic Table143 Questions
Exam 3: Chemical Compounds113 Questions
Exam 4: Chemical Composition144 Questions
Exam 5: Chemical Reactions and Equations129 Questions
Exam 6: Quantities in Chemical Reactions133 Questions
Exam 7: Electron Structure of the Atom133 Questions
Exam 8: Chemical Bonding124 Questions
Exam 9: The Gaseous State121 Questions
Exam 10: The Liquid and Solid States118 Questions
Exam 11: Solutions119 Questions
Exam 12: Reaction Rates and Chemical Equilibrium110 Questions
Exam 13: Acids and Bases137 Questions
Exam 14: Oxidation-Reduction Reactions120 Questions
Exam 15: Nuclear Chemistry106 Questions
Exam 16: Organic Chemistry129 Questions
Exam 17: Biochemistry116 Questions
Select questions type
Consider the reaction between acetylene, C2H2, and oxygen in a welding torch: 2C2H2(g)+ 5O2(g)→ 4CO2(g)+ 2H2O(g)If 5.4 moles of acetylene react with sufficient oxygen, how many grams of CO2 should form?
Free
(Multiple Choice)
4.9/5  (46)
(46)
Correct Answer:
C
When sodium sulfate, Na2SO4, dissolves in water, the ions that are formed for each formula unit that dissolves are:
Free
(Multiple Choice)
4.7/5  (34)
(34)
Correct Answer:
C
The coefficients of a balanced equation can be understood to represent either relative numbers of molecules or moles.
Free
(True/False)
4.9/5  (36)
(36)
Correct Answer:
True
Phosphine, PH3, a reactive and poisonous compound, reacts with oxygen as follows: 4PH3(g)+ 8O2(g)→ P4O10(s)+ 6H2O(g)If 9.2 moles of phosphine react with sufficient oxygen, how many moles of P4O10 should form?
(Multiple Choice)
4.8/5  (40)
(40)
The figure shows a molecular-level diagram of reactant molecules for the reaction 2H2(g)+ O2(g)→ 2H2O(l) 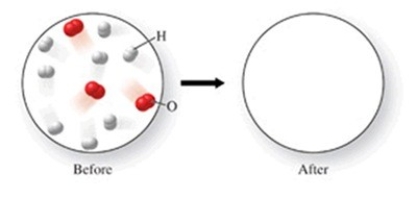 List the number and formulas of the molecules that should be present after the reaction takes place.
List the number and formulas of the molecules that should be present after the reaction takes place.
(Multiple Choice)
4.9/5  (40)
(40)
Consider the reaction between sodium metal and chlorine gas to form sodium chloride (table salt): 2Na(s)+ Cl2(g)→ 2NaCl(s)If the mass of the sodium solid increases by 0.500 g, what mass of sodium metal should have reacted?
(Multiple Choice)
4.8/5  (36)
(36)
Consider the reaction between hydrogen and oxygen gases to form water: 2H2(g)+ O2(g)→ 2H2O(l)Which of the following is not conserved in this reaction?
(Multiple Choice)
4.7/5  (40)
(40)
When copper reacts with sulfur at high temperature, copper(I)sulfide is formed. 2Cu(s)+ S(s)→ Cu2S(s)If the mass of the Cu2S formed is 1.17 g, what mass of copper should have reacted?
(Multiple Choice)
4.8/5  (30)
(30)
The combustion of octane is described by the following balanced equation: 2C8H18(l)+ 25O2(g)→ 16CO2(g)+ 18H2O(g)If 50.0 g of each reactant are available to react, how much CO2 should form? Report your answer with the correct number of significant figures and correct units.
(Short Answer)
4.8/5  (39)
(39)
Aluminum reacts with oxygen according to the following reaction: 4Al(s)+ 3O2(g)→ 2Al2O3(s)If 24 moles of aluminum are combined with 12 moles of oxygen, how many moles of Al2O3 should form?
(Multiple Choice)
4.9/5  (40)
(40)
If you have eight bicycle wheels and five frames, how many bikes could you build (assuming that each bike requires one frame and two wheels), and what would be left over?
(Multiple Choice)
4.9/5  (41)
(41)
The limiting reactant in a chemical reaction is always the reactant which is present in the least amount in terms of mass.
(True/False)
4.8/5  (38)
(38)
Consider the following specific heats of metals. 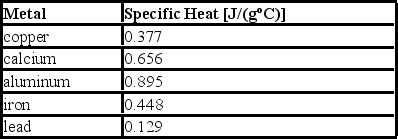 If the same amount of heat is added to 50.0 g of each of these metals, all at the same initial temperature, which metal will have the lowest final temperature?
If the same amount of heat is added to 50.0 g of each of these metals, all at the same initial temperature, which metal will have the lowest final temperature?
(Multiple Choice)
4.9/5  (42)
(42)
Consider the reaction between sodium metal and chlorine gas to form sodium chloride (table salt): 2Na(s)+ Cl2(g)→ 2NaCl(s)If 3.6 moles of chlorine react with sufficient sodium, how many grams of sodium chloride should form?
(Multiple Choice)
4.9/5  (42)
(42)
An equal quantity of heat is transferred to 10.0 g samples of different substances. Given their specific heat values, rank the substances in order from least to greatest final temperature. 
(Multiple Choice)
4.9/5  (33)
(33)
When phosphorus reacts with chlorine, phosphorus trichloride is formed according to the following equation: ___P4(s)+ ___Cl2(g)→ ___PCl3(l)(unbalanced)Balance the equation and determine how many grams of chlorine would be required to react with 21.2 g of phosphorus.
(Multiple Choice)
4.9/5  (40)
(40)
What is the heat change when a 53.5 g sample of water [Cwater = 4.184 J/(g°C)] is cooled from 98.0°C to 23.2°C?
(Multiple Choice)
4.9/5  (38)
(38)
A 3.50 g sample of rice was burned in a bomb calorimeter containing 1980 g of water. The temperature of the water increased from 22.75°C to 28.88°C. How much heat, in joules, did the rice sample release when it burned? [Cwater = 4.184 J/(g°C)]
(Multiple Choice)
4.9/5  (38)
(38)
Showing 1 - 20 of 133
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)