Exam 6: Quantities in Chemical Reactions
Exam 1: Matter and Energy121 Questions
Exam 2: Atoms, Ions, and the Periodic Table143 Questions
Exam 3: Chemical Compounds113 Questions
Exam 4: Chemical Composition144 Questions
Exam 5: Chemical Reactions and Equations129 Questions
Exam 6: Quantities in Chemical Reactions133 Questions
Exam 7: Electron Structure of the Atom133 Questions
Exam 8: Chemical Bonding124 Questions
Exam 9: The Gaseous State121 Questions
Exam 10: The Liquid and Solid States118 Questions
Exam 11: Solutions119 Questions
Exam 12: Reaction Rates and Chemical Equilibrium110 Questions
Exam 13: Acids and Bases137 Questions
Exam 14: Oxidation-Reduction Reactions120 Questions
Exam 15: Nuclear Chemistry106 Questions
Exam 16: Organic Chemistry129 Questions
Exam 17: Biochemistry116 Questions
Select questions type
A carton of low-fat yogurt says it has 1.70 x 102 Calories. What is the equivalent amount of energy in units of joules?
(Multiple Choice)
4.9/5  (33)
(33)
In the process of obtaining lead from PbS, or galena, the galena is "roasted" (heated in the presence of oxygen), so that the following reaction occurs: 2PbS(s)+ 3O2(g)→ 2PbO(s)+ 2SO2(g)If 35.2 g of PbS is mixed with 15.5 g of oxygen, how many grams of PbO should form?
(Multiple Choice)
4.8/5  (43)
(43)
Consider the reaction between hydrogen and oxygen gases to form water: 2H2(g)+ O2(g)→ 2H2O(l)If 8.5 moles of oxygen react with sufficient hydrogen, how many grams of water should form?
(Multiple Choice)
4.9/5  (45)
(45)
When a substance cools from a high temperature to a low temperature, its heat change value will have a negative sign.
(True/False)
4.9/5  (40)
(40)
Which of the following is the best (simplest)balanced equation to represent the chemical reaction shown in the figure on any scale? 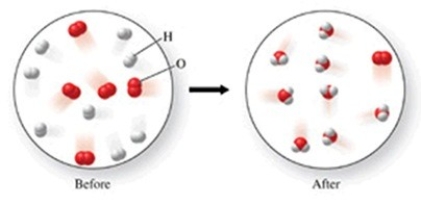
(Multiple Choice)
4.8/5  (43)
(43)
When acetylene, C2H2, a fuel used in welding, is burned in air, two molecules of acetylene combine with five oxygen molecules to form four CO2 molecules and two H2O molecules. Select the statement below that is incorrect in regard to this reaction.
(Multiple Choice)
4.8/5  (36)
(36)
A 5.00 g sample of a brownie was burned in a bomb calorimeter containing 2025 g of water. The temperature of the water increased from 23.50°C to 33.47°C. How much heat, in joules, did the brownie release when it burned? [Cwater = 4.184 J/(g°C)]
(Multiple Choice)
4.9/5  (39)
(39)
The number of moles of reactant molecules must always equal the number of moles of product molecules in a balanced equation.
(True/False)
4.9/5  (39)
(39)
When the mixture of molecules shown in the molecular-level image undergoes complete reaction, all of these molecules are converted to products. Which of the following reactions could this represent? 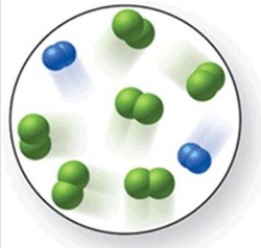
(Multiple Choice)
4.9/5  (42)
(42)
Iron metal reacts with hydrochloric acid as follows: 2Fe(s)+ 6HCl(aq)→ 2FeCl3(aq)+ 3H2(g)If 22.4 g of iron react with excess HCl, and 59.4 g of FeCl3 are collected, what is the percent yield of Al2(SO4)3?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following best describes an exothermic reaction?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following is the best (simplest)balanced equation to represent the chemical reaction shown in the figure on any scale? 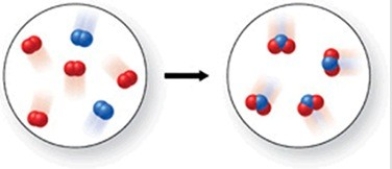
(Multiple Choice)
4.9/5  (34)
(34)
The specific heat of a substance is the amount of heat energy that is needed to cause the substance to melt.
(True/False)
4.9/5  (40)
(40)
A calorie used by nutritionists, 1 Calorie, is equal to 1000 cal or 1 kcal used by chemists.
(True/False)
4.9/5  (30)
(30)
Consider the reaction between acetylene, C2H2, and oxygen in a welding torch: 2C2H2(g)+ 5O2(g)→ 4CO2(g)+ 2H2O(g)Which of the following is not conserved in this reaction?
(Multiple Choice)
4.7/5  (35)
(35)
What mass (in grams)of SF6 should be produced by the following reaction if 7.00 g of sulfur is mixed with 9.00 g of fluorine? S + 3F2 → SF6
(Multiple Choice)
4.7/5  (25)
(25)
Iron metal reacts with hydrochloric acid as follows: 2Fe(s)+ 6HCl(aq)→ 2FeCl3(aq)+ 3H2(g)If 35.6 g of iron react with excess HCl, and 98.6 g of FeCl3 are collected, what is the percent yield of FeCl3?
(Multiple Choice)
4.8/5  (43)
(43)
When carbon dioxide is formed from its elements, 393.5 kJ of energy is released. Convert this energy to units of calories.
(Multiple Choice)
4.8/5  (39)
(39)
How much heat energy would be needed to raise the temperature of a 32.0 g sample of gold [C = 0.129 (J/g°C)] from 21.8°C to 75.0°C?
(Multiple Choice)
4.9/5  (44)
(44)
Showing 21 - 40 of 133
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)