Exam 6: Quantities in Chemical Reactions
Exam 1: Matter and Energy121 Questions
Exam 2: Atoms, Ions, and the Periodic Table143 Questions
Exam 3: Chemical Compounds113 Questions
Exam 4: Chemical Composition144 Questions
Exam 5: Chemical Reactions and Equations129 Questions
Exam 6: Quantities in Chemical Reactions133 Questions
Exam 7: Electron Structure of the Atom133 Questions
Exam 8: Chemical Bonding124 Questions
Exam 9: The Gaseous State121 Questions
Exam 10: The Liquid and Solid States118 Questions
Exam 11: Solutions119 Questions
Exam 12: Reaction Rates and Chemical Equilibrium110 Questions
Exam 13: Acids and Bases137 Questions
Exam 14: Oxidation-Reduction Reactions120 Questions
Exam 15: Nuclear Chemistry106 Questions
Exam 16: Organic Chemistry129 Questions
Exam 17: Biochemistry116 Questions
Select questions type
All of the following may change during a chemical reaction except
(Multiple Choice)
4.7/5  (44)
(44)
An aqueous solution containing 15.0 g of NaOH is mixed with an aqueous solution containing 15.0 g of H2SO4. Is sodium hydroxide or sulfuric acid the limiting reactant?
(Short Answer)
4.8/5  (50)
(50)
Given that 4NH3(g)+ 5O2(g)→ 4NO(g)+ 6H2O(g), if 6.3 moles of NH3 react with sufficient oxygen, how many moles of NO should form?
(Multiple Choice)
4.7/5  (37)
(37)
When the mixture of molecules shown in the molecular-level image undergoes complete reaction, all of these molecules are converted to products. Which of the following reactions could this represent? 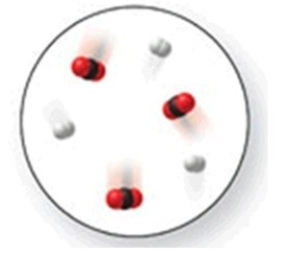
(Multiple Choice)
4.9/5  (43)
(43)
When sulfur dioxide is formed from its elements, 296.8 kJ of energy is released. Convert this energy to units of calories.
(Multiple Choice)
4.9/5  (32)
(32)
When 3.0 mol CaCl2 dissolves in water, how many moles of ions are in solution?
(Multiple Choice)
4.8/5  (30)
(30)
In a bomb calorimeter, the bomb itself, the water surrounding it, and the thermometer would be considered part of the "system."
(True/False)
4.8/5  (31)
(31)
Nitrogen and hydrogen react together to form ammonia according to the equation: ___N2(g)+ ___H2(g)→ ___NH3(g)(unbalanced)Balance the equation, and determine how many grams of hydrogen would be required to react with 25.2 g of nitrogen.
(Multiple Choice)
4.8/5  (28)
(28)
When fats or other foods are burned in a bomb calorimeter, the calorimeter absorbs heat, so the reaction is endothermic.
(True/False)
4.9/5  (30)
(30)
Consider the reaction N2(g)+ 2O2(g)→ 2NO2(g). The molecular image represents a mixture of N2(g)and O2(g)just before reaction occurs. What is the limiting reactant, and how much of the excess reactant remains after the reaction is complete? The image contains 3 N2 molecules and 9 O2 molecules. 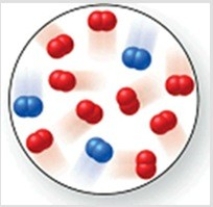
(Multiple Choice)
4.9/5  (34)
(34)
When phosphorus reacts with chlorine, phosphorus trichloride is formed according to the equation: ___P4(s)+ ___Cl2(g)→ ___PCl3(l)(unbalanced)Balance the equation and determine how many grams of phosphorus reactant would be required to produce 25.0 g of phosphorus trichloride, assuming there is sufficient chlorine available.
(Multiple Choice)
4.9/5  (29)
(29)
Consider the following reaction: 3NO2(g)+ H2O(l)→ 2HNO3(l)+ NO(g)How many moles of NO2 are required to react with 1.50 moles of H2O to form 3.00 moles of HNO3?
(Multiple Choice)
4.9/5  (45)
(45)
In photosynthesis, plants convert carbon dioxide and water into glucose, C6H12O6, according to the reaction: 6CO2(g)+ 6H2O(l)→ 6O2(g)+ C6H12O6(g)If 75.2 g of glucose are obtained when 131 g of CO2 react in the presence of excess H2O, what is the percent yield of the reaction? Report your answer with the correct number of significant figures.
(Short Answer)
4.9/5  (37)
(37)
Aluminum metal reacts with sulfuric acid according to the following equation: 2Al(s)+ 3H2SO4(aq)→ Al2(SO4)3(s)+ 3H2(g)If 12.9 g of aluminum reacts with excess sulfuric acid, and 62.4 g of Al2(SO4)3 are collected, what is the percent yield of Al2(SO4)3?
(Multiple Choice)
4.9/5  (49)
(49)
When mixed, solutions of aluminum nitrate, Al(NO3)3, and ammonium carbonate, (NH4)2CO3, will form a precipitate of aluminum carbonate, Al2(CO3)3. The balanced equation is 2Al(NO3)3(aq)+ 3(NH4)2CO3(aq)→ Al2(CO3)3 (s)+ 6NH4NO3(aq)Which of the following statements regarding this reaction is incorrect?
(Multiple Choice)
4.9/5  (39)
(39)
In the process of obtaining lead from PbS, or galena, the galena is "roasted" (heated in the presence of oxygen), so that the following reaction occurs: 2PbS(s)+ 3O2(g)→ 2PbO(s)+ 2SO2(g)If 50.0 g of PbS are mixed with 25.0 g of oxygen, how many grams of PbO should form?
(Multiple Choice)
4.9/5  (46)
(46)
If you wish to make sandwiches which consist of two slices of bread, one ham slice, and three pickle slices, how many sandwiches could you make if you have 12 slices of bread, five slices of ham, and 20 pickle slices, and what would be left over?
(Multiple Choice)
4.8/5  (44)
(44)
When ammonium carbonate, (NH4)2CO3, dissolves in water, the ions that are formed for each formula unit that dissolves are:
(Multiple Choice)
4.8/5  (37)
(37)
When blue copper(II)sulfate pentahydrate is heated, it decomposes according to the equation given below, forming light blue copper(II)sulfate and water vapor. CuSO4·5H2O(s)→ CuSO4(s)+ 5H2O(g)If a 5.00 g sample of CuSO4·5H2O is heated, what mass of CuSO4 will be formed when the reaction is complete? Report your answer with the correct number of significant figures and correct units.
(Short Answer)
4.7/5  (35)
(35)
When calculating the percent yield for a reaction, the only information necessary is the mass of each reactant.
(True/False)
4.8/5  (34)
(34)
Showing 61 - 80 of 133
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)