Exam 1: Covalent Bonding and Shapes of Molecules
Exam 1: Covalent Bonding and Shapes of Molecules114 Questions
Exam 2: Acids and Bases47 Questions
Exam 3: Alkanes and Cycloalkanes60 Questions
Exam 4: Alkenes and Alkynes58 Questions
Exam 6: Chirality: the Handedness of Molecules59 Questions
Exam 7: Haloalkanes58 Questions
Exam 8: Alcohols, Ethers, and Thiols55 Questions
Exam 9: Benzene and Its Derivatives48 Questions
Exam 10: Amines55 Questions
Exam 11: Infrared and Nuclear Magnetic Resonance Spectroscopy117 Questions
Exam 12: Aldehydes and Ketones58 Questions
Exam 13: Carboxylic Acids59 Questions
Exam 14: Functional Derivatives of Carboxylic Acids59 Questions
Exam 15: Enolate Anions60 Questions
Exam 16: Organic Polymer Chemistry58 Questions
Exam 17: Carbohydrates57 Questions
Exam 18: Amino Acids and Proteins59 Questions
Exam 19: Lipids60 Questions
Exam 20: Nucleic Acids58 Questions
Exam 21: The Organic Chemistry of Metabolism62 Questions
Select questions type
Which compound has the most exothermic (most negative) heat of hydrogenation? 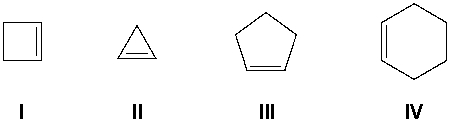
(Multiple Choice)
4.7/5  (39)
(39)
Complete the following reaction by providing the starting material and reagent and solvent. Do not include lone pairs in the answer. 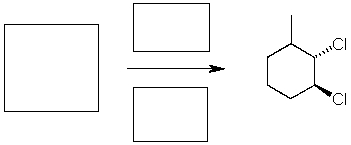
(Essay)
4.7/5  (36)
(36)
Atoms of the group 2A elements react by losing two electrons to achieve a noble gas configuration.
(True/False)
4.9/5  (28)
(28)
Which is the correct Lewis structure for acetic acid (CH3CO2H)?
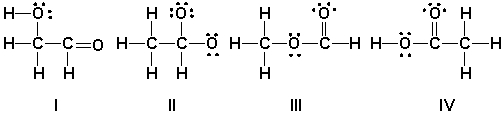
(Multiple Choice)
4.7/5  (46)
(46)
Using the VSEPR model, predict which molecules have bond angles of about 109°.
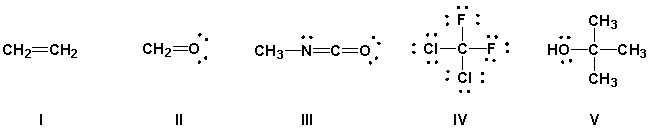
(Multiple Choice)
4.8/5  (34)
(34)
Which atom is described by the electron configuration 1s22s22p63s23p5?
(Multiple Choice)
4.9/5  (40)
(40)
Which is the major product from the reaction of 1,2-dimethylcyclohexene with Br2? 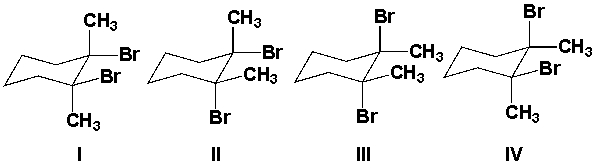
(Multiple Choice)
4.8/5  (39)
(39)
The following molecule contains the _________ and __________ functional groups.
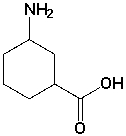
(Short Answer)
4.7/5  (29)
(29)
Complete the following reaction by providing the necessary catalyst and solvent/reagent. 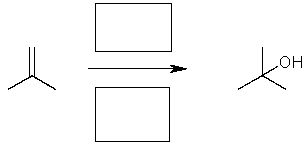 Type missing reagents and solvents through the sing plus.
Type missing reagents and solvents through the sing plus.
(Essay)
4.9/5  (41)
(41)
Which is the major product from acid catalyzed hydration of 2-methyl-2-pentene?
(Multiple Choice)
4.8/5  (48)
(48)
1-Cyclohexyl-1-butyne reacts with molecular hydrogen under pressure in the presence of a platinum catalyst. What reaction product is formed? 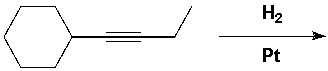
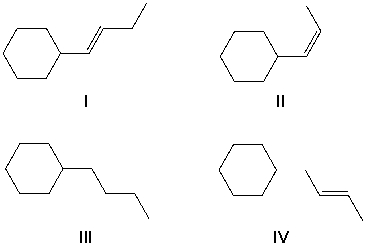
(Multiple Choice)
4.7/5  (38)
(38)
______ is the number of valence electrons for a neutral bromine atom.
(Short Answer)
4.9/5  (38)
(38)
Which reagent, in addition to 2-methyl-1-butene, leads to the formation of an alcohol?
HBr/CH2Cl2 I
BH3, followed by H2O2/NaOH/H2O II
H2/Pt III
Br2 IV
H+/H2O V
(Multiple Choice)
4.8/5  (41)
(41)
The carbon has the correct orbital hybridization in which structures?
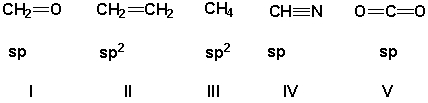
(Multiple Choice)
4.9/5  (37)
(37)
Which is the major product from the reaction of propene with BH3 followed by NaOH/H2O2?
(Multiple Choice)
4.7/5  (29)
(29)
Which of the three molecules testosterone, methadone and hydrocodone contains a hydroxyl group.
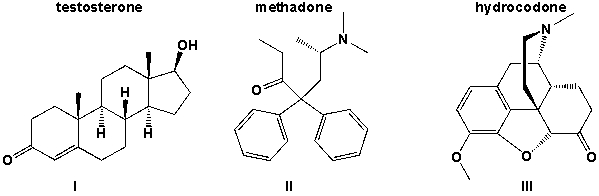
(Multiple Choice)
4.9/5  (41)
(41)
Showing 81 - 100 of 114
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)