Exam 1: Covalent Bonding and Shapes of Molecules
Exam 1: Covalent Bonding and Shapes of Molecules114 Questions
Exam 2: Acids and Bases47 Questions
Exam 3: Alkanes and Cycloalkanes60 Questions
Exam 4: Alkenes and Alkynes58 Questions
Exam 6: Chirality: the Handedness of Molecules59 Questions
Exam 7: Haloalkanes58 Questions
Exam 8: Alcohols, Ethers, and Thiols55 Questions
Exam 9: Benzene and Its Derivatives48 Questions
Exam 10: Amines55 Questions
Exam 11: Infrared and Nuclear Magnetic Resonance Spectroscopy117 Questions
Exam 12: Aldehydes and Ketones58 Questions
Exam 13: Carboxylic Acids59 Questions
Exam 14: Functional Derivatives of Carboxylic Acids59 Questions
Exam 15: Enolate Anions60 Questions
Exam 16: Organic Polymer Chemistry58 Questions
Exam 17: Carbohydrates57 Questions
Exam 18: Amino Acids and Proteins59 Questions
Exam 19: Lipids60 Questions
Exam 20: Nucleic Acids58 Questions
Exam 21: The Organic Chemistry of Metabolism62 Questions
Select questions type
Which Lewis structures are correct?
HINT: Perform a total valence count and check formal charges.
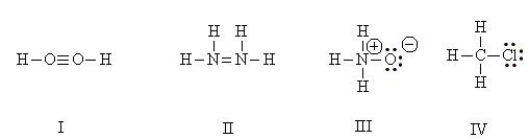
(Multiple Choice)
4.9/5  (42)
(42)
Using Markovnikov's rule, predict the position of the Cl atom in the major product from the reaction of 1-methylcyclohexene with HCl. 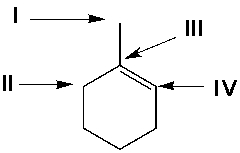
(Multiple Choice)
4.9/5  (35)
(35)
Which of the three molecules aspirin, paracetamol and ibuprofen contains a carboxyl group?
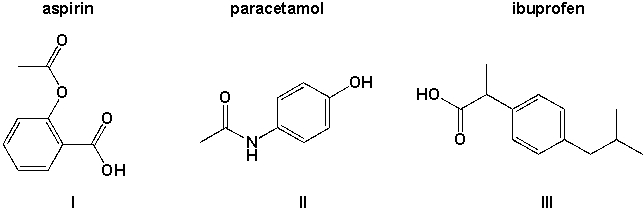
(Multiple Choice)
4.9/5  (32)
(32)
Using the VSEPR model, predict which molecules have bond angles of about 120°.
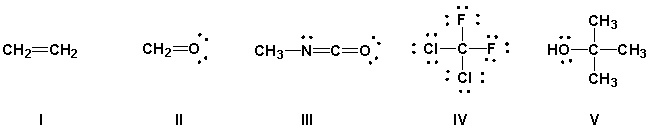
(Multiple Choice)
4.8/5  (43)
(43)
The following carbocations are listed in increasing order of stability (least tofirst). 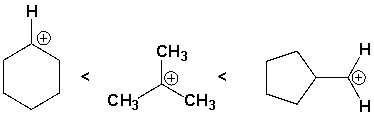
(True/False)
4.8/5  (35)
(35)
The hydroboration and subsequent oxidation of 1-heptene leads to which of the following products? 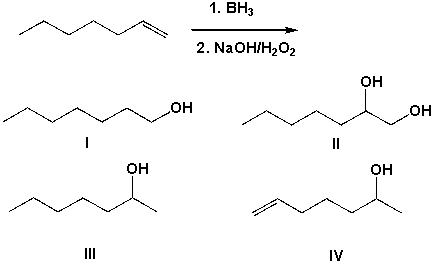
(Multiple Choice)
4.7/5  (38)
(38)
But-1-yn-1-ylcyclohexane reacts with molecular hydrogen under pressure in the presence of a Lindlar catalyst. What reaction product is formed? 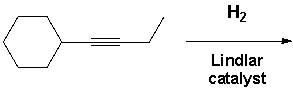
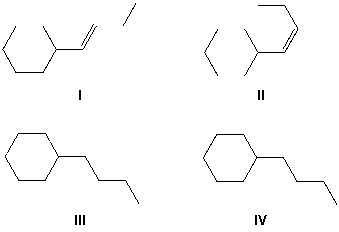
(Multiple Choice)
4.9/5  (42)
(42)
According to VSEPR model, what is your prediction for the arrangement of electron pairs for CH3-?
(Multiple Choice)
4.8/5  (25)
(25)
The following molecule contains the _________ and __________ functional groups.
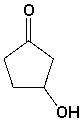
(Short Answer)
4.8/5  (31)
(31)
Using the VSEPR model, predict which species have bond angles of about 109°.
HINT: Assume that the charges are correct. Add the missing lone pairs before applying the VSEPR theory!
(Multiple Choice)
4.9/5  (37)
(37)
Showing 101 - 114 of 114
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)