Exam 1: Chemistry: the Central Science
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
A large pizza has a diameter of 15 inches. Express this diameter in centimeters. (1 in = 2.54 cm)
(Multiple Choice)
4.9/5  (39)
(39)
When applying the scientific method, a model or theory should be based on experimental data.
(True/False)
4.8/5  (39)
(39)
Dry ice (carbon dioxide) changes from a solid to a gas at -78.5°C. What is this temperature in °F?
(Multiple Choice)
4.9/5  (39)
(39)
Explain the difference between quantitative measurements and qualitative measurements.
(Essay)
4.9/5  (37)
(37)
The mass of a sample is 550 milligrams. Which of the following expresses that mass in kilograms?
(Multiple Choice)
4.9/5  (39)
(39)
If two numbers are added together, one which has 2 digits after the decimal point and the other which has 1 digit after the decimal point, explain how to round the answer.
(Essay)
4.7/5  (49)
(49)
Which of the following does not have a uniform composition throughout?
(Multiple Choice)
4.8/5  (33)
(33)
The average distance between the Earth and the Moon is 240,000 miles. Express this distance in meters. (1 mi = 1609 m)
(Multiple Choice)
4.9/5  (31)
(31)
The city of Los Angeles is now approximately 2400 miles south of Anchorage, Alaska. It is moving slowly northward as the San Andreas fault slides along. If Los Angeles is to arrive near Anchorage in 76 million years, at what average rate will it have to move in mm per month? (1 mi = 1609 m)
(Multiple Choice)
4.8/5  (38)
(38)
If you have a graduated cylinder containing 15.5 mL of water and this volume increases to 95.2 mL after a piece of metal with a mass of 7.95 g is dropped into the graduated cylinder, then what is the density of the metal?
(Multiple Choice)
4.8/5  (38)
(38)
Select the answer that expresses the result of this calculation with the correct number of significant figures. 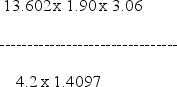
(Multiple Choice)
5.0/5  (38)
(38)
Which of the following properly expresses 0.01234 in scientific notation?
(Multiple Choice)
4.9/5  (33)
(33)
A flask has a mass of 78.23 g when empty and 593.63 g when filled with water. When the same flask is filled with concentrated sulfuric acid, H2SO4, its mass is 1026.57 g. What is the density of concentrated sulfuric acid? (Assume water has a density of 1.00 g/cm3 at the temperature of the measurement.)
(Multiple Choice)
4.8/5  (48)
(48)
Showing 41 - 60 of 133
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)