Exam 1: Chemistry: the Central Science
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
What is the volume of a container that contains 14.3 g of a substance having a density of 0.988 g/cm3?
(Multiple Choice)
4.8/5  (29)
(29)
The boiling point for liquid helium is 4.0 K. What is the temperature in degrees Fahrenheit?
(Multiple Choice)
4.9/5  (45)
(45)
A ________ mixture does not have a uniform composition throughout.
(Multiple Choice)
4.7/5  (31)
(31)
If a patient in the hospital is running a temperature of 39.5°C, what is this in degrees Fahrenheit?
(Multiple Choice)
4.7/5  (39)
(39)
________ is the term used to indicate a measuring device is accurate.
(Short Answer)
4.8/5  (45)
(45)
A(n)________ is a substance that cannot be separated into simpler substances by chemical means.
(Short Answer)
4.9/5  (43)
(43)
What is the length of the box, using the proper number of significant figures and units? 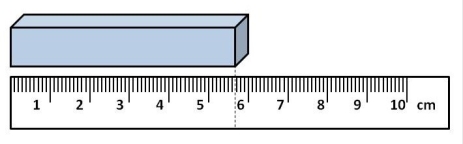
(Multiple Choice)
4.8/5  (30)
(30)
When applying the scientific method, it is important to avoid any form of hypothesis.
(True/False)
4.9/5  (41)
(41)
A(n) ________ is a substance composed of atoms of two or more elements chemically united in fixed proportions.
(Short Answer)
4.8/5  (30)
(30)
Explain the difference between a heterogeneous mixture and a homogeneous mixture.
(Essay)
4.9/5  (40)
(40)
Some molecules move with speeds approaching the "escape velocity" from Earth, which is 7.0 miles per second. What is this speed in cm/h? (1 mi = 1609 m)
(Multiple Choice)
4.9/5  (35)
(35)
50.0 grams of acetic acid are required for an experiment. What volume, in milliliters, of a 1.105 g/cm3 acetic acid solution must be measured for the experiment?
(Multiple Choice)
4.8/5  (27)
(27)
Which of the following correctly expresses 52.068881 in scientific notation, rounded to three significant figures?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following is a 'substance' according to the definition given in your textbook?
(Multiple Choice)
4.8/5  (33)
(33)
Showing 101 - 120 of 133
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)