Exam 11: Acids and Bases
Exam 1: Chemistry in Our Lives40 Questions
Exam 2: Chemistry and Measurements70 Questions
Exam 3: Matter and Energy77 Questions
Exam 4: Atoms and Elements83 Questions
Exam 5: Nuclear Chemistry64 Questions
Exam 6: Ionic and Molecular Compounds83 Questions
Exam 7: Chemical Reactions and Quantities96 Questions
Exam 8: Gases84 Questions
Exam 9: Solutions84 Questions
Exam 10: Reaction Rates and Chemical Equilibrium52 Questions
Exam 11: Acids and Bases72 Questions
Exam 12: Introduction to Organic Chemistry: Hydrocarbons105 Questions
Exam 13: Alcohols, Phenols, Thiols, and Ethers49 Questions
Exam 14: Aldehydes and Ketones44 Questions
Exam 15: Carbohydrates54 Questions
Exam 16: Carboxylic Acids and Esters55 Questions
Exam 17: Lipids70 Questions
Exam 18: Amines and Amides60 Questions
Exam 19: Amino Acids and Proteins66 Questions
Exam 20: Enzymes and Vitamins68 Questions
Exam 21: Nucleic Acids and Protein Synthesis55 Questions
Exam 22: Metabolic Pathways for Carbohydrates58 Questions
Exam 23: Metabolism and Energy Production51 Questions
Exam 24: Metabolic Pathways for Lipids and Amino Acids56 Questions
Select questions type
A patient with alkalosis has a blood pH of 7.60 . What is the 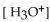 of her blood plasma?
of her blood plasma?
(Multiple Choice)
4.9/5  (45)
(45)
In a neutralization reaction, how many moles of 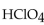 react with 1 mole of
react with 1 mole of 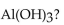
(Multiple Choice)
5.0/5  (31)
(31)
Write the proper acid dissociation expression for the ionization of acetic acid, 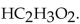
(Essay)
4.9/5  (36)
(36)
What is the molarity of a KOH solution if 25.0 mL neutralizes 35.0 mL of a 0.200 M HCl solution?
(Multiple Choice)
4.8/5  (41)
(41)
A 10.0 mL of 0.121 M 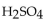 is neutralized by 17.1 mL of KOH solution. The molarity of the KOH solution is
is neutralized by 17.1 mL of KOH solution. The molarity of the KOH solution is
(Multiple Choice)
4.9/5  (31)
(31)
The neutralization reaction between 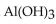 and
and 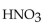 produces the salt with the formula
produces the salt with the formula
(Multiple Choice)
4.8/5  (37)
(37)
Showing 21 - 40 of 72
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)