Exam 1: Structure and Bonding:acids and Bases
Exam 1: Structure and Bonding:acids and Bases41 Questions
Exam 2: Alkanes: the Nature of Organic Compounds44 Questions
Exam 3: Alkenes and Alkynes: the Nature of Organic Reactions40 Questions
Exam 4: Reactions of Alkenes and Alkynes44 Questions
Exam 5: Aromatic Compounds52 Questions
Exam 6: Sterechemistry at Tetrahedral Centers39 Questions
Exam 7: Organohalides: Nucleophilic Substitutions and Eliminations40 Questions
Exam 8: Alcohols, Phenols, Ethers, and Their Sulfur Analogs36 Questions
Exam 9: Aldehydes and Ketones: Nucleophilic Addition Reactions32 Questions
Exam 10: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution Reactions63 Questions
Exam 11: Carbonyl Alpha-Substitution Reactions and Condensation Reactions38 Questions
Exam 12: Amines32 Questions
Exam 13: Structure Determination65 Questions
Exam 14: Biomolecules: Carbohydrates48 Questions
Exam 15: Biomolecules: Amino Acids, Peptides, and Proteins50 Questions
Exam 16: Biomolecules: Lipids and Nucleic Acids50 Questions
Exam 17: The Organic Chemistry of Metabolic Pathways40 Questions
Select questions type
Draw a Lewis structure for each of the following.
a)hydroxylammonium ion: +NH3OH.
b)azide ion: (N3-)
(Essay)
4.9/5  (30)
(30)
Draw the structure for CCl2F2 using solid, wedged, and dashed lines to show the tetrahedral geometry.
(Essay)
4.7/5  (31)
(31)
Instructions: Refer to the following equation to answer the question(s) below.  -Refer to instructions. The strongest Brønsted-Lowry acid in the equation is indicated by letter _____.
-Refer to instructions. The strongest Brønsted-Lowry acid in the equation is indicated by letter _____.
(Short Answer)
4.8/5  (44)
(44)
The structure of urea is shown below. Fill in any nonbonding valence electrons that are missing from the line-bond structure. 
(Essay)
4.8/5  (43)
(43)
Identify the reactants and product in the reaction below as acids or bases and specify whether they are Lewis and/or Brønsted-Lowry. 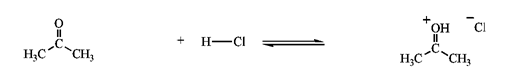
(Essay)
4.9/5  (38)
(38)
Convert the skeletal drawing of the pharmaceutical Vioxx into a molecular formula. 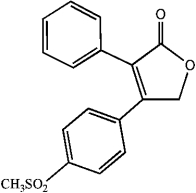
(Short Answer)
4.7/5  (32)
(32)
Instructions: Write valid Lewis (electron-dot) structures for each formula below. Show all electrons as dots and show all nonbonding electrons.
-Write:
CH3CH2OH ethanol
(Essay)
4.8/5  (40)
(40)
Instructions: Use the convention / + and the crossed arrow  to show the direction of the expected polarity of the indicated bonds in the following compound(s).
-Refer to instructions. The C-F bond in fluorobenzene,
to show the direction of the expected polarity of the indicated bonds in the following compound(s).
-Refer to instructions. The C-F bond in fluorobenzene, 
(Essay)
4.7/5  (45)
(45)
Instructions: Refer to the following equation to answer the question(s) below.  -Refer to instructions. The strongest Brønsted-Lowry base in the equation is indicated by letter _____.
-Refer to instructions. The strongest Brønsted-Lowry base in the equation is indicated by letter _____.
(Short Answer)
4.9/5  (45)
(45)
Specify the hybridization of each carbon atom of limonene, a natural product present in citrus fruits, and thujone, which is derived from wormwood, a traditional component of the notorious liquor, Absinthe. 
(Essay)
4.8/5  (38)
(38)
Instructions: Determine the hybridization for the indicated atoms in each structure below. 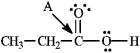
 -Refer to instructions. The hybridization of carbon atom A is _____.
-Refer to instructions. The hybridization of carbon atom A is _____.
(Short Answer)
4.8/5  (30)
(30)
Instructions: Determine the hybridization for the indicated atoms in each structure below. 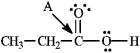
 -Refer to instructions. The hybridization of carbon atom B is _____.
-Refer to instructions. The hybridization of carbon atom B is _____.
(Short Answer)
4.8/5  (37)
(37)
Consider the formation of an sp2 hybrid orbital. Which of the following is true?
(Multiple Choice)
4.8/5  (30)
(30)
Instructions: Indole is pleasant smelling in highly dilute solutions and has been used in perfumery. Use the structure of indole, below, to answer the following question(s).  -Refer to instructions. Indole can function as a Lewis base in the presence of strong acid. Formulate a reaction, using a generic acid (HA), showing electron flow with arrows, that demonstrates this reactivity of indole.
-Refer to instructions. Indole can function as a Lewis base in the presence of strong acid. Formulate a reaction, using a generic acid (HA), showing electron flow with arrows, that demonstrates this reactivity of indole.
(Essay)
4.7/5  (34)
(34)
Instructions: Propose a structure for a molecule that meets the following description.
-Refer to instructions. Contains only one sp3 hybridized carbon and two sp2 hybridized carbons.
(Essay)
4.7/5  (35)
(35)
Instructions: Consider the species below to answer the following question.
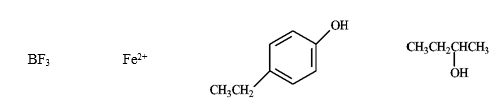 -Refer to instructions. Which of the following would be common to all?
-Refer to instructions. Which of the following would be common to all?
(Multiple Choice)
4.8/5  (34)
(34)
Draw all the lone pairs (nonbonding valence electrons) on the structure of phosgene, a poisonous gas once used as a chemical warfare agent. 
(Essay)
4.8/5  (35)
(35)
Showing 21 - 40 of 41
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)