Exam 20: Quantum Physics of Molecules and Solids
Exam 1: Electric Fields46 Questions
Exam 2: Gausss Law44 Questions
Exam 3: Electric Potential49 Questions
Exam 4: Energy and Capacitance38 Questions
Exam 5: Current and Resistance33 Questions
Exam 6: Direct-Current Circuits49 Questions
Exam 7: Magnetic Fields45 Questions
Exam 8: Magnetic Forces50 Questions
Exam 9: Faradays Law49 Questions
Exam 10: Inductance23 Questions
Exam 11: Alternating-Current Circuits50 Questions
Exam 12: Electromagnetic Waves40 Questions
Exam 13: The Nature of Light and the Principles of Ray Optics38 Questions
Exam 14: Image Formation45 Questions
Exam 15: Wave Optics43 Questions
Exam 16: Diffraction Patterns and Polarisation44 Questions
Exam 17: Quantisation and Wave-Particle Duality41 Questions
Exam 18: Introduction to Quantum Mechanics43 Questions
Exam 19: Atomic Physics49 Questions
Exam 20: Quantum Physics of Molecules and Solids46 Questions
Exam 21: Nuclei and Radioactivity48 Questions
Exam 22: Particle Physics34 Questions
Select questions type
The rotation spectrum of the HCl molecule has been observed in the far infrared, around 50 * 10-6 m.The spacing between successive lines in the spectrum corresponds to radiation of wavelength equal to 5 microns (1 m = 10-6 m).Determine the frequency of the photon associated with this transition.
(Multiple Choice)
4.9/5  (47)
(47)
In the hydrogen molecule, H2, the separation between the protons is 10-10 m.If the molecule is in its first rotational energy state, what is the angular velocity of the molecule about its centre of mass?
(Short Answer)
4.8/5  (41)
(41)
A molecule makes a transition from the J = 1 to the J = 0 rotational energy state.The wavelength of the emitted photon is 2.6* 10-3 m.What is the moment of inertia of the molecule (in kg .m2)?
(Multiple Choice)
4.7/5  (32)
(32)
The diagram below shows the distance between the nuclei, pA and pB, and the electrons, e1 and e2, in a hydrogen molecule.We would expect the electrostatic potential energy of this molecule to be: 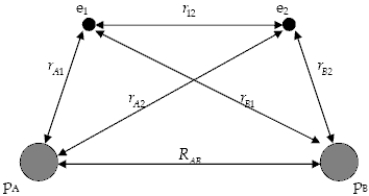
(Multiple Choice)
4.7/5  (26)
(26)
The fundamental frequency of CO is 6.42 *1013 Hz.If the atomic masses are 12 u and 16 u (1 u = 1.66 *10-27 kg), find the force constant (in N/m) for the diatomic molecule.
(Multiple Choice)
4.9/5  (38)
(38)
The rotation spectrum of the HCl molecule suggests a photon in the far infrared (around 5.0 *10-6 m) can excite the first rotational level.From this data, the moment of inertia of the HCl molecule (in kg . m2) is:
(Multiple Choice)
4.8/5  (32)
(32)
The rotational kinetic energy of a diatomic molecule can take the form:
(Multiple Choice)
4.8/5  (34)
(34)
In the Lennard-Jones model of the hydrogen molecule, the potential is given by .In this model, the minimum internuclear separation, r0, is: 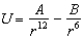
(Multiple Choice)
4.9/5  (40)
(40)
The energy released when an atom takes an electron is called:
(Multiple Choice)
4.8/5  (36)
(36)
When a molecule jumps from a rotational energy level characterised by the rotational quantum number J to one characterised by J + 1, the change in energy, EJ + 1 - EJ, is:
(Multiple Choice)
4.8/5  (35)
(35)
The difference between donor and acceptor atoms in a doped semiconductor is that:
(Multiple Choice)
5.0/5  (43)
(43)
The energy gap for germanium is 0.670 eV at room temperature.What wavelength must a photon have (in nm) to excite the electron to the conduction band?
(Multiple Choice)
4.9/5  (31)
(31)
The dissociation energy of the hydrogen molecule is approximately 5 eV.What is the temperature of a monatomic molecule whose kinetic energy is equal to 5.000 eV?
(Multiple Choice)
4.8/5  (35)
(35)
Because HF, hydrogen fluoride, is a covalent gaseous molecule at room temperature, we might reasonably expect that at room temperature HCl, hydrogen chloride, is:
(Multiple Choice)
4.9/5  (32)
(32)
When a molecule jumps from a rotational energy level characterised by the rotational quantum number J to one characterised by J- 1, the difference in energy of levels J and J -1, EJ - EJ - 1, is:
(Multiple Choice)
4.8/5  (36)
(36)
In comparing vibrational and rotational levels in molecules, we find that the energy separation between adjacent energy levels is:
(Multiple Choice)
4.7/5  (44)
(44)
The moment of inertia of a CO molecule is 1.46 *10-46 kg . m2.What is the wavelength of the photon emitted if a rotational transition occurs from the J = 3 to the J = 2 state?
(Multiple Choice)
4.8/5  (41)
(41)
To find the number of electrons per unit volume with energy between E and E + dE in a metal we must multiply the number of allowed states per unit volume with energy E by:
(Multiple Choice)
4.8/5  (34)
(34)
The Fermi energy of a metal at a temperature T is 7.0 eV.What is the average energy (in eV) of a conduction electron at that temperature?
(Multiple Choice)
4.8/5  (37)
(37)
The energy gap for a semiconductor is 1.25 eV.Of the frequencies given below, what is the minimum frequency photon than can move an electron from the valence band to the conduction band?
(Multiple Choice)
4.8/5  (33)
(33)
Showing 21 - 40 of 46
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)