Exam 7: Solutions and Colloids
Exam 1: Matter, Measurements, and Calculations101 Questions
Exam 2: Atoms and Molecules96 Questions
Exam 3: Electronic Structure and the Periodic Law91 Questions
Exam 4: Forces Between Particles94 Questions
Exam 5: Chemical Reactions95 Questions
Exam 6: The States of Matter95 Questions
Exam 7: Solutions and Colloids98 Questions
Exam 8: Reaction Rates and Equilibrium92 Questions
Exam 9: Acids, Bases, and Salts92 Questions
Exam 10: Radioactivity and Nuclear Processes93 Questions
Exam 11: Organic Compounds: Alkanes92 Questions
Exam 12: Unsaturated Hydrocarbons94 Questions
Exam 13: Alcohols, Phenols, and Ethers92 Questions
Exam 14: Aldehydes and Ketones93 Questions
Exam 15: Carboxylic Acids and Esters94 Questions
Exam 16: Amines and Amides92 Questions
Exam 17: Carbohydrates93 Questions
Exam 18: Lipids96 Questions
Exam 19: Proteins97 Questions
Exam 20: Enzymes94 Questions
Exam 21: Nucleic Acids and Protein Synthesis98 Questions
Exam 22: Nutrition and Energy for Life94 Questions
Exam 23: Carbohydrate Metabolism95 Questions
Exam 24: Lipid and Amino Acid Metabolism99 Questions
Exam 25: Body Fluids91 Questions
Select questions type
Express the following concentration of solution in terms of molarity: 3.00 L of solution contains 1.75 mol of solute.
(Multiple Choice)
4.7/5  (43)
(43)
A 100 mL sample of a saturated CaSO4 solution is evaporated to dryness. The water solvent all evaporates and leaves behind a solid residue of CaSO4 that weighs 0.23 g. The CaSO4 would be classified as
(Multiple Choice)
4.9/5  (38)
(38)
When solid NaOH is dissolved in water, the solution becomes hot. The solution process is
(Multiple Choice)
4.9/5  (35)
(35)
How does the solubility of a gas change with change in pressure at a constant temperature?
(Multiple Choice)
4.9/5  (42)
(42)
Changes in boiling point, freezing point, and vapor pressure are
(Multiple Choice)
4.8/5  (38)
(38)
A solution is made by dissolving a small amount of salt in a beaker of water. The water is referred to as the
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following pairs correctly represent similar functions for a solution component and a colloid component?
(Multiple Choice)
4.8/5  (46)
(46)
A solution is made by combining 4.00 g of sugar and 100 mL of water (density = 1.00 g\mL). What is the concentration in % w\w?
(Multiple Choice)
4.9/5  (49)
(49)
What is the molarity of a solution containing 0.325 moles of solute in 250 mL of solution?
(Multiple Choice)
4.7/5  (33)
(33)
Suppose a solution contains 200 g water and 15 g glucose. Which of the following statements is true?
(Multiple Choice)
4.7/5  (33)
(33)
When will carbon dioxide in a carbonated soft drink dissolve best?
(Multiple Choice)
4.8/5  (39)
(39)
You have a patient who is suffering from the "bends". What gas is in excess in the blood?
(Multiple Choice)
4.8/5  (51)
(51)
A solution is prepared at 75 °C by dissolving 50.0 g of A in 100 g of water. Which of the following correctly classifies this solution based on the solubility chart for A given below? 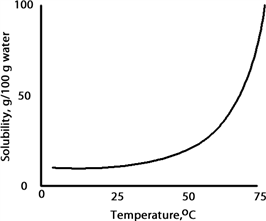
(Multiple Choice)
4.8/5  (35)
(35)
Showing 81 - 98 of 98
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)