Exam 7: Solutions and Colloids
Exam 1: Matter, Measurements, and Calculations101 Questions
Exam 2: Atoms and Molecules96 Questions
Exam 3: Electronic Structure and the Periodic Law91 Questions
Exam 4: Forces Between Particles94 Questions
Exam 5: Chemical Reactions95 Questions
Exam 6: The States of Matter95 Questions
Exam 7: Solutions and Colloids98 Questions
Exam 8: Reaction Rates and Equilibrium92 Questions
Exam 9: Acids, Bases, and Salts92 Questions
Exam 10: Radioactivity and Nuclear Processes93 Questions
Exam 11: Organic Compounds: Alkanes92 Questions
Exam 12: Unsaturated Hydrocarbons94 Questions
Exam 13: Alcohols, Phenols, and Ethers92 Questions
Exam 14: Aldehydes and Ketones93 Questions
Exam 15: Carboxylic Acids and Esters94 Questions
Exam 16: Amines and Amides92 Questions
Exam 17: Carbohydrates93 Questions
Exam 18: Lipids96 Questions
Exam 19: Proteins97 Questions
Exam 20: Enzymes94 Questions
Exam 21: Nucleic Acids and Protein Synthesis98 Questions
Exam 22: Nutrition and Energy for Life94 Questions
Exam 23: Carbohydrate Metabolism95 Questions
Exam 24: Lipid and Amino Acid Metabolism99 Questions
Exam 25: Body Fluids91 Questions
Select questions type
Which of the following tend to stabilize colloids and prevent suspended particles from settling?
(Multiple Choice)
4.8/5  (37)
(37)
When preparing an aqueous solution of a salt, one must always be able to observe some undissolved material to be sure that it is saturated.
(True/False)
4.9/5  (39)
(39)
The solubility of gases in water increases with increasing temperature.
(True/False)
4.8/5  (49)
(49)
Match the types of colloids with their examples.
Premises:
Milk
Responses:
Solid foam
Sol
Foam
Correct Answer:
Premises:
Responses:
(Matching)
4.9/5  (40)
(40)
Match the types of colloids with their examples.
Premises:
Ink
Responses:
Sol
Solid foam
Aerosol
Correct Answer:
Premises:
Responses:
(Matching)
4.8/5  (38)
(38)
One test to determine if a mixture is a true solution or a colloid is ____.
(Multiple Choice)
4.8/5  (33)
(33)
Attractive forces between solute and solvent molecules are an important factor in solution formation.
(True/False)
4.8/5  (35)
(35)
When a patient's blood electrolyte levels are evaluated, sodium, chloride and bicarbonate ions are commonly measured and the difference in the total positive and negative charges calculated. This difference is called the
(Multiple Choice)
5.0/5  (28)
(28)
Which of the following correctly arranges 1.00 M solutions of the strong electrolytes in order of increasing boiling point (lowest to highest)?
(Multiple Choice)
4.8/5  (32)
(32)
The solubility of a substance can be measured in grams substance dissolved per liter of water. This is the same as expressing solubility in moles per liter.
(True/False)
4.8/5  (31)
(31)
Which of the following pass through both osmotic and dialysis membranes?
(Multiple Choice)
5.0/5  (34)
(34)
The cleaning action of soaps and detergents is attributable to
(Multiple Choice)
4.8/5  (37)
(37)
How many moles of Na2CO3 are needed to react with 750 mL of 0.250 M H2SO4 solution? Na2CO3 + H2SO4 → Na2SO4 + CO2 + H2O
(Multiple Choice)
4.8/5  (38)
(38)
Light scattering is an effective way to distinguish between true solutions and colloidal dispersions.
(True/False)
4.8/5  (34)
(34)
The following equation can be used when C represents either a M or %(w\w)concentration.
CcVc = CdVd
(True/False)
4.7/5  (29)
(29)
You discover that your roommate has left a half-full bottle of cola on the kitchen counter without replacing the cap - AGAIN!. Not surprisingly, the cola is now flat (all carbonation is gone). Why did this happen?
(Multiple Choice)
4.9/5  (49)
(49)
In the colloid known as mayonnaise, the dispersed phase is _____ .
(Multiple Choice)
4.9/5  (36)
(36)
Weight-volume percentage solutions must be made in 100 mL increments.
(True/False)
4.8/5  (27)
(27)
Citric acid, a natural food preservative, accounts for the tartness of citrus fruits. It is shown below. About 730 g of this material can be dissolved in water, making a liter of solution. However, only about 1.5% of it dissociates. As such, it would be considered a _____. 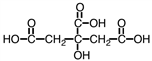
(Multiple Choice)
4.8/5  (36)
(36)
Showing 61 - 80 of 98
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)