Exam 1: Introduction to Chemistry
Exam 1: Introduction to Chemistry95 Questions
Exam 2: Atoms, Molecules, and Ions132 Questions
Exam 3: Equations, the Mole, and Chemical Formulas186 Questions
Exam 4: Chemical Reactions in Solution107 Questions
Exam 5: Thermochemistry78 Questions
Exam 6: The Gaseous State140 Questions
Exam 7: Electronic Structure86 Questions
Exam 8: The Periodic Table: Structure and Trends90 Questions
Exam 9: Chemical Bonds119 Questions
Exam 10: Molecular Structure and Bonding Theories133 Questions
Exam 11: Liquids and Solids100 Questions
Exam 12: Solutions199 Questions
Exam 13: Chemical Kinetics148 Questions
Exam 14: Chemical Equilibrium212 Questions
Exam 15: Solutions of Acids and Bases179 Questions
Exam 16: Reactions Between Acids and Bases98 Questions
Exam 17: Chemical Thermodynamics106 Questions
Exam 18: Electrochemistry112 Questions
Exam 19: Transition Metals, Coordination Chemistry and Metallurgy73 Questions
Exam 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases41 Questions
Exam 21: Nuclear Chemistry89 Questions
Exam 22: Organic Chemistry and Biochemistry175 Questions
Select questions type
Which of the following is correctly classified as a hypothesis?
(Multiple Choice)
4.7/5  (42)
(42)
What length in millimeters can be reported for the rectangle below?
(Note that the ruler measures in centimeters.) 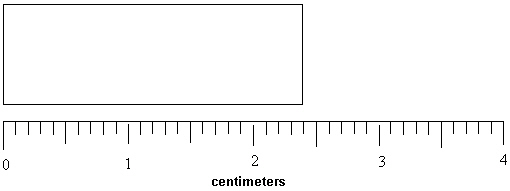
(Multiple Choice)
4.8/5  (39)
(39)
A person weighs 83.2 kg. What is this person's weight in pounds?
(1)00 pound = 454 grams)
(Multiple Choice)
4.9/5  (41)
(41)
What is the speed of an automobile given in miles per second (miles/sec) that is traveling 80. kilometer per hour (km/hour)?
(1 km = 0.621 miles; 1 hour = 60 min; 1 min = 60 sec)
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following measurements is considered a combination of one of the seven base units from which all other measurements can be made?
(Multiple Choice)
4.8/5  (33)
(33)
The atomic radius of a beryllium atom is 111 pm. Express this distance in inches.
(Multiple Choice)
4.7/5  (39)
(39)
The speed limit is 70 miles per hour on some stretches of the interstate. What is this same speed in meters per second, (m/s)?
(1 mile = 1.60 km)
(Multiple Choice)
4.9/5  (46)
(46)
What digit(s) is(are) considered the digit of uncertainty in the following measurement?
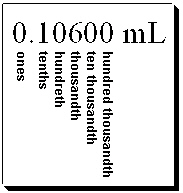
(Multiple Choice)
5.0/5  (34)
(34)
The speed limit on the interstate in Virginia is 65 miles per hour. What is this speed in kilometers per second?
(1 km = 0.621 miles, 1 hour = 60 minutes and 1 minute = 60 seconds)
(Multiple Choice)
4.7/5  (42)
(42)
Exhibit 1-1 Consider the following figure that is a blow-up view of a region of a 50 mL burette to answer the following question(s). 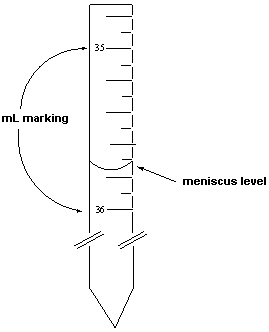 The density of ethanol was measured in two steps. The first step involved measuring its volume using a burette. The mass of this volume was then measured.
-Refer to Exhibit 1-1. If the mass of the ethanol delivered was 28.217 grams, what is the density of this liquid reported to the correct number of significant digits?
The density of ethanol was measured in two steps. The first step involved measuring its volume using a burette. The mass of this volume was then measured.
-Refer to Exhibit 1-1. If the mass of the ethanol delivered was 28.217 grams, what is the density of this liquid reported to the correct number of significant digits?
(Multiple Choice)
4.7/5  (40)
(40)
What is the mass of 25.0 mL of an oil if its density is 0.843 g/mL?
(Multiple Choice)
4.7/5  (35)
(35)
How can "oil and vinegar" be classified?
(Two phases are present.)
(Multiple Choice)
4.9/5  (34)
(34)
What is the temperature given by the thermometer below written with the proper number of significant digits?

(Multiple Choice)
4.7/5  (34)
(34)
What prefix is used to indicate a base unit multiplied by 10 - 6?
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following is an example of a homogeneous mixture ?
I. Water and dissolved salt
II. Water and sand
III. Carbonated water (soda) and ice
(Multiple Choice)
4.9/5  (43)
(43)
A carpenter must lay a floor that covers 1500 ft2. What is this area in cm2?
(1 foot = 12 inches and 1 inch = 2.54 cm)
(Multiple Choice)
4.8/5  (34)
(34)
The smallest bone in the human body, which is in the ear, has a mass of 0.0030 grams. What is this mass in pounds?
(1 pound = 454 grams)
(Multiple Choice)
4.8/5  (32)
(32)
Showing 21 - 40 of 95
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)