Exam 1: Introduction to Chemistry
Exam 1: Introduction to Chemistry95 Questions
Exam 2: Atoms, Molecules, and Ions132 Questions
Exam 3: Equations, the Mole, and Chemical Formulas186 Questions
Exam 4: Chemical Reactions in Solution107 Questions
Exam 5: Thermochemistry78 Questions
Exam 6: The Gaseous State140 Questions
Exam 7: Electronic Structure86 Questions
Exam 8: The Periodic Table: Structure and Trends90 Questions
Exam 9: Chemical Bonds119 Questions
Exam 10: Molecular Structure and Bonding Theories133 Questions
Exam 11: Liquids and Solids100 Questions
Exam 12: Solutions199 Questions
Exam 13: Chemical Kinetics148 Questions
Exam 14: Chemical Equilibrium212 Questions
Exam 15: Solutions of Acids and Bases179 Questions
Exam 16: Reactions Between Acids and Bases98 Questions
Exam 17: Chemical Thermodynamics106 Questions
Exam 18: Electrochemistry112 Questions
Exam 19: Transition Metals, Coordination Chemistry and Metallurgy73 Questions
Exam 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases41 Questions
Exam 21: Nuclear Chemistry89 Questions
Exam 22: Organic Chemistry and Biochemistry175 Questions
Select questions type
A comfortable temperature for bath water is 95 ° F. What temperature is this on the Kelvin scale?
(Multiple Choice)
4.8/5  (35)
(35)
How many gallons are present in 43.7 Liters?
(1 gallon = 4 quarts and 1 quart = 0.946 L)
(Multiple Choice)
4.7/5  (31)
(31)
Exhibit 1-1 Consider the following figure that is a blow-up view of a region of a 50 mL burette to answer the following question(s). 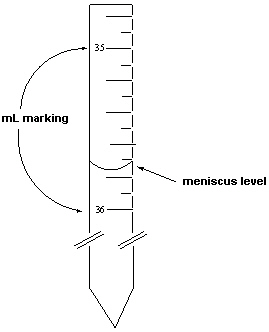 The density of ethanol was measured in two steps. The first step involved measuring its volume using a burette. The mass of this volume was then measured.
-Refer to Exhibit 1-1. In the reported measurement above for the volume of the ethanol displaced from the burette (given in milliliters), what digit is the digit of uncertainty ?
The density of ethanol was measured in two steps. The first step involved measuring its volume using a burette. The mass of this volume was then measured.
-Refer to Exhibit 1-1. In the reported measurement above for the volume of the ethanol displaced from the burette (given in milliliters), what digit is the digit of uncertainty ?
(Multiple Choice)
4.9/5  (39)
(39)
Choose the best answer to the following math problem ( with the proper number of significant digits and rounded correctly ). (12.3432 - 8.84)×22.48 = ??
(Multiple Choice)
4.8/5  (32)
(32)
What is the density of mercury when reported to the proper number of significant digits and in scientific notation if a sample is found to have a mass of 524.5 g and occupies a volume of 38.72 ml?
(Multiple Choice)
5.0/5  (34)
(34)
Which measurements below have four significant digits?
Measurement
I. 0.023 mL
II. 2300 mL
III. 1700 . mL
IV. 0.004050 mL
(Multiple Choice)
4.8/5  (33)
(33)
Which form of substances listed below cannot be separated into simpler substances by chemical or physical means?
(Multiple Choice)
4.8/5  (35)
(35)
What volume of gold has a mass of 25.0 grams?
(Density of gold = 19.30 g/mL)
(Multiple Choice)
4.8/5  (40)
(40)
The definition "stable pure substance that cannot be separated into simpler substances by chemical means" would best fit which term listed below?
(Multiple Choice)
4.8/5  (42)
(42)
The dimensions of the following cube were measured. What would be the volume of this cube expressed to the proper number of significant digits?
(Volume = l w d ) 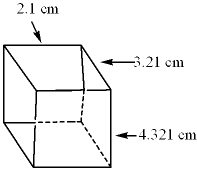
(Multiple Choice)
4.9/5  (34)
(34)
Of the three properties listed below, which ones are intensive properties?
I. mass
II. density
III. color
(Multiple Choice)
4.8/5  (35)
(35)
"A 33 g sample of red liquid at its freezing point of 260 K had a density of 0.926 g/mL." Which of the properties given in the previous statement is extensive ?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following matched pairs of elemental names and chemical symbols is properly labeled?
I. Calcium, C
II. Plutonium, Pt
III. Silicon, Si
IV. Iron, I
V. Nitrogen, N
(Multiple Choice)
4.9/5  (44)
(44)
The area of a telescope lens is 5773 mm2. What is this area given in square feet, ft2?
(1 inch = 2.54 cm)
(Multiple Choice)
5.0/5  (31)
(31)
Showing 61 - 80 of 95
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)