Exam 8: The Periodic Table: Structure and Trends
Exam 1: Introduction to Chemistry95 Questions
Exam 2: Atoms, Molecules, and Ions132 Questions
Exam 3: Equations, the Mole, and Chemical Formulas186 Questions
Exam 4: Chemical Reactions in Solution107 Questions
Exam 5: Thermochemistry78 Questions
Exam 6: The Gaseous State140 Questions
Exam 7: Electronic Structure86 Questions
Exam 8: The Periodic Table: Structure and Trends90 Questions
Exam 9: Chemical Bonds119 Questions
Exam 10: Molecular Structure and Bonding Theories133 Questions
Exam 11: Liquids and Solids100 Questions
Exam 12: Solutions199 Questions
Exam 13: Chemical Kinetics148 Questions
Exam 14: Chemical Equilibrium212 Questions
Exam 15: Solutions of Acids and Bases179 Questions
Exam 16: Reactions Between Acids and Bases98 Questions
Exam 17: Chemical Thermodynamics106 Questions
Exam 18: Electrochemistry112 Questions
Exam 19: Transition Metals, Coordination Chemistry and Metallurgy73 Questions
Exam 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases41 Questions
Exam 21: Nuclear Chemistry89 Questions
Exam 22: Organic Chemistry and Biochemistry175 Questions
Select questions type
How many unpaired electrons does the ground state of Fe3+ have?
(Multiple Choice)
4.8/5  (40)
(40)
Given the three statements below, pick the best answer.
I. Cl has a higher electron affinity than P.
II. The ionization energy for Si is greater than that for P+.
III. The electron affinity for O is greater than that for Se.
(Multiple Choice)
4.7/5  (40)
(40)
Which of the following series of atoms are arranged in order of increasing size?
(Multiple Choice)
4.8/5  (36)
(36)
Which element listed below would most likely have the following series of ionization energies?
IE1 IE2 IE3 IE4 IE5 IE6 0.80 MJ/mol 2.43 MJ/mol 3.66 MJ/mol 6.22 MJ/mol 37.83 MJ/mol 47.28 MJ/mol
(Multiple Choice)
4.8/5  (38)
(38)
Arrange the following elements in order of increasing atomic radii . Barium, Calcium, Bromine
(Multiple Choice)
4.9/5  (46)
(46)
Three reactions are shown below. Pick the best answer.
I. 2 Li + 2 H2O→2 LiOH + H2
II. 6 Li + N2→2 Li3N
III. Cl2 + H2→2 HCl
(Multiple Choice)
5.0/5  (33)
(33)
The ground state electron configuration of the ion, P+, may be represented as:
(Multiple Choice)
4.8/5  (30)
(30)
The ground state of which species below has the most unpaired electrons?
(Multiple Choice)
4.7/5  (36)
(36)
Of the three species, which would be isoelectronic with O?
I. N+
II. F -
III. Ne2 -
(Multiple Choice)
4.8/5  (31)
(31)
Which element would be expected to have the most favorable electron affinity ?
(Multiple Choice)
4.7/5  (51)
(51)
Listed below are three rankings of atoms or ions in order of increasing ionization energy. Pick the correct answer.
I. B
II. C
III. Al
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following processes listed below represents the experiment for measuring an electron affinity ?
("E" represents a neutral E lement, "M" represents a M etal cation and "X" represents a nonmetal anion)
(Multiple Choice)
4.7/5  (43)
(43)
Exhibit 8-1 The following question(s) relate to the diagram below:
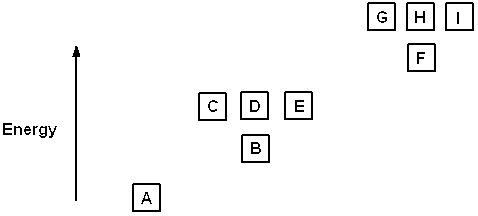 Refer to Exhibit 8-1. The three quantum numbers that describe the state labeled F are:
Refer to Exhibit 8-1. The three quantum numbers that describe the state labeled F are:
(Multiple Choice)
4.7/5  (51)
(51)
Listed below are three rankings of atoms or ions in order of increasing ionization energies. Pick the best answer.
I. Li
II. P
III. Al
(Multiple Choice)
4.7/5  (33)
(33)
Arrange the following species in order of increasing radii . N3 - , F - , Ne, Na+1, Mg2+
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following processes listed below represents the experiment for measuring an ionization energy ?
("E" represents a neutral E lement, "M" represents a M etal cation and "X" represents a nonmetal anion)
(Multiple Choice)
4.9/5  (42)
(42)
Showing 21 - 40 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)