Exam 8: The Periodic Table: Structure and Trends
Exam 1: Introduction to Chemistry95 Questions
Exam 2: Atoms, Molecules, and Ions132 Questions
Exam 3: Equations, the Mole, and Chemical Formulas186 Questions
Exam 4: Chemical Reactions in Solution107 Questions
Exam 5: Thermochemistry78 Questions
Exam 6: The Gaseous State140 Questions
Exam 7: Electronic Structure86 Questions
Exam 8: The Periodic Table: Structure and Trends90 Questions
Exam 9: Chemical Bonds119 Questions
Exam 10: Molecular Structure and Bonding Theories133 Questions
Exam 11: Liquids and Solids100 Questions
Exam 12: Solutions199 Questions
Exam 13: Chemical Kinetics148 Questions
Exam 14: Chemical Equilibrium212 Questions
Exam 15: Solutions of Acids and Bases179 Questions
Exam 16: Reactions Between Acids and Bases98 Questions
Exam 17: Chemical Thermodynamics106 Questions
Exam 18: Electrochemistry112 Questions
Exam 19: Transition Metals, Coordination Chemistry and Metallurgy73 Questions
Exam 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases41 Questions
Exam 21: Nuclear Chemistry89 Questions
Exam 22: Organic Chemistry and Biochemistry175 Questions
Select questions type
How many unpaired electrons does the ground state of S have?
(Multiple Choice)
4.7/5  (32)
(32)
Exhibit 8-1 The following question(s) relate to the diagram below:
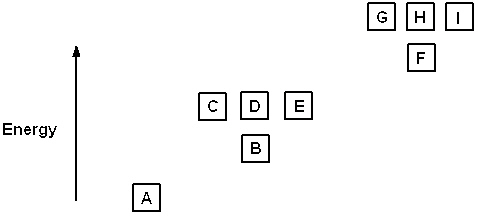 Refer to Exhibit 8-1. The three quantum numbers that describe the orbital labeled B are:
Refer to Exhibit 8-1. The three quantum numbers that describe the orbital labeled B are:
(Multiple Choice)
4.9/5  (37)
(37)
Given the three statements below, pick the best answer.
I. Be is larger than Li
II. Be+ is larger than Li
III. O2 - is larger than F -
(Multiple Choice)
4.8/5  (23)
(23)
Given the three statements below, pick the best answer.
I. The Zeff for the highest energy electron in Be is higher than the Zeff for the highest energy electron for N.
II. The second ionization energy for Mg is greater than the first ionization energy of Na.
III. The species O2 - , Ne, and Na are isoelectronic.
(Multiple Choice)
4.9/5  (36)
(36)
Arrange the following elements in order of increasing ionization energy. O, Mg, He
(Multiple Choice)
4.7/5  (41)
(41)
What is the value of n (principal quantum number) for a valence electron in a ground state Ca atom?
(Multiple Choice)
4.7/5  (35)
(35)
Three series of atoms are listed below in order of increasing size. Pick the best answer.
I. Li
II. B
III. Cl
(Multiple Choice)
4.8/5  (40)
(40)
Based upon the electron affinity trend, which group of atoms is most likely to acquire an additional electron?
(Multiple Choice)
4.8/5  (39)
(39)
What is the highest energy subshell that is occupied in the ground state of an atom of Bi (at. no. = 83)?
(Multiple Choice)
4.9/5  (35)
(35)
Arrange the elements Al, Na, and P in order of increasing atomic radii based upon their position on the periodic table.
(Multiple Choice)
4.7/5  (37)
(37)
How many unpaired electrons does the ground state of the species Al+ have?
(Multiple Choice)
4.8/5  (40)
(40)
Which series shown below is correctly arranged in order of increasing ionization energy?
(Multiple Choice)
4.9/5  (38)
(38)
Given the three statements below, pick the best answer.
I. For the H atom, the energy states are dependent only on n.
II. Knowing the values of n, 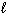 ,
, 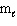 specifies an orbital.
III. The highest energy electron for a K atom is located in a 4s orbital.
specifies an orbital.
III. The highest energy electron for a K atom is located in a 4s orbital.
(Multiple Choice)
5.0/5  (39)
(39)
Which of the following series of atoms are arranged in order of decreasing size?
(Multiple Choice)
4.8/5  (44)
(44)
Which element listed below has the most exothermic electron affinity?
(Multiple Choice)
4.8/5  (26)
(26)
Showing 41 - 60 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)