Exam 12: Oxidation-Reduction Reactions
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
What is the correct coefficient for Na2MnO4 in the balanced equation for the air oxidation of manganese dioxide to sodium manganate in the presence of sodium carbonate?
MnO2(s) + Na2CO3(aq) + O2(g) CO2(g) + Na2MnO4(aq)
(Multiple Choice)
4.7/5  (37)
(37)
What is the coefficient for sulfur in the following redox reaction?
S(s) + KClO3(s) SO2(g) + KCl(s) + bang!
(Multiple Choice)
4.9/5  (32)
(32)
refer to the following reaction which occurs in basic solution.
CrO42- + PH3 Cr(OH)4- + P4
-Which of the following are oxidizing agents in the forward and reverse directions, respectively?
(Multiple Choice)
4.9/5  (27)
(27)
For the following reaction, identify the oxidizing agents in the forward and reverse directions, respectively..
P4(s) + HNO3(aq) H3PO4(aq) + NO(g)
(Multiple Choice)
4.9/5  (42)
(42)
 Determining Oxidation Numbers
-Determine the oxidation number of arsenic in the anion, HAsO42 - .
Determining Oxidation Numbers
-Determine the oxidation number of arsenic in the anion, HAsO42 - .
(Multiple Choice)
4.7/5  (44)
(44)
A current of 2.0 amps passed through a molten solution of the salt MCl2 for a period of 30 minutes produces 1.32 g of Cl2 gas and 0.453 g of M. Which of the following metals is present in this compound?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following is true about the reaction below?
2VO2+ (aq) + Br2(s) + 2 H2O(l) 2VO2+(aq) + 2 Br - (aq) + 4H+
(Multiple Choice)
4.8/5  (42)
(42)
What is the ratio of the weight of Cl2 produced at the anode to the weight of Al produced at the cathode when 5 moles of electrons are passed through a molten sample of AlCl3?
(Multiple Choice)
5.0/5  (46)
(46)
Redox Reactions in Basic Solutions
refer to the following reaction which occurs in basic solution:
OCl-(aq) ClO2-(aq) + Cl2(aq)
-How many OH- ions are involved in the simplest balanced equation for this reaction?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following statements about this reaction is correct?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following pairs of ions can't coexist in aqueous solution under standard-state conditions because a spontaneous redox reaction occurs?
(Multiple Choice)
4.9/5  (33)
(33)
What is the oxidation state of the tin atom in an unknown salt if 14.8 grams of tin are plated out when a current of 40 amps is passed through a molten solution of this salt for 10 minutes?
(Multiple Choice)
4.8/5  (39)
(39)
Redox Reactions in Basic Solutions
refer to the following reaction which occurs in basic solution:
OCl-(aq) ClO2-(aq) + Cl2(aq)
-What is the net effect on the OH- ion concentration in this reaction?
(Multiple Choice)
4.8/5  (40)
(40)
Use a table of standard reduction potentials to determine which of the following is the strongest reducing agent.
(Multiple Choice)
4.8/5  (35)
(35)
The following equation, when balanced, involves a total of ___ electrons.
ClO3-(aq) + I2(aq) + H2O(l) IO3-(aq) + Cl-(aq) + H+(aq)
(Multiple Choice)
5.0/5  (30)
(30)
Which of the following statements about the electrolysis of an aqueous solution of NaCl is true?
(Multiple Choice)
4.9/5  (39)
(39)
Balance the following redox reaction. Clearly show the two balanced half-reactions and indicate which represents oxidation and which reduction.
H2O2(aq) + NO(g) + H+(aq) NO3-(aq)
(Essay)
4.8/5  (43)
(43)
In the electrolysis of NaBr(aq), which of the following products is least likely to be found?
(Multiple Choice)
4.9/5  (30)
(30)
A current of 1.5 amps is applied to a 1.00 L solution of 0.100 M hydrochloric acid for 1.0 hr. What is the pH of the solution after electrolysis is complete?
(Short Answer)
4.7/5  (38)
(38)
refer to the following incomplete, unbalanced equation
Cr2O72-(aq) + NO(g)
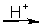 Cr3+(aq) + NO3-(aq)
-What is the coefficient of the NO in the balanced equation?
Cr3+(aq) + NO3-(aq)
-What is the coefficient of the NO in the balanced equation?
(Multiple Choice)
4.8/5  (42)
(42)
Showing 61 - 80 of 81
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)