Exam 15: Oxidation and Reduction
Exam 1: The Science of Chemistry62 Questions
Exam 2: Numbers From Measurements99 Questions
Exam 3: Unit Systems and Dimensional Analysis83 Questions
Exam 4: Basic Concepts About Matter75 Questions
Exam 5: Atoms, Molecules, Formulas, and Subatomic Particles87 Questions
Exam 6: Electronic Structure and Chemical Periodicity92 Questions
Exam 7: Chemical Bonds92 Questions
Exam 8: Chemical Nomenclature97 Questions
Exam 9: Chemical Calculations: the Mole Concept and Chemical Formulas93 Questions
Exam 10: Chemical Calculations Involving Chemical Equations68 Questions
Exam 11: States of Matter65 Questions
Exam 12: Gas Laws78 Questions
Exam 13: Solutions64 Questions
Exam 14: Acids, Bases and Salts65 Questions
Exam 15: Oxidation and Reduction70 Questions
Exam 16: Reaction Rates and Chemical Equilibrium52 Questions
Exam 17: Nuclear Chemistry69 Questions
Select questions type
In the following reaction: 2 Al (s) + 3 I2 (s) 2 AlI3 (s) which species is the oxidizing agent?
(Multiple Choice)
4.7/5  (37)
(37)
In the reactions below, which reaction correctly identifies the anode reaction during the discharging (normal usage) of a lead storage battery cell?
(Multiple Choice)
4.9/5  (41)
(41)
In a galvanic cell, the cathode is the electrode at which ________ occurs?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following statements is not true of a redox reaction?
(Multiple Choice)
4.8/5  (35)
(35)
Indicate whether each of the following formula unit or net ionic equations is or is not a redox reaction by writing the word
-N2(g) + 3 H2(g) 2 NH3(g) ____________________
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following is a correctly balanced oxidation half-reaction?
(Multiple Choice)
4.8/5  (32)
(32)
The proper assignment of oxidation numbers to the elements in Na2CrO4 would be ________.
(Multiple Choice)
4.9/5  (37)
(37)
In the following reaction H2SO4 is ________.
H2SO4 + HI I2 + SO2 + H2O
(Multiple Choice)
4.9/5  (42)
(42)
Indicate whether each of the following formula unit or net ionic equations is or is not a redox reaction by writing the word
-2 Al(s) + 3 FeO(s) 3 Fe(s) + Al2O3(s) ____________________
(Multiple Choice)
4.9/5  (39)
(39)
Identify which substance is oxidized and which substance is reduced in the following chemical reaction.
4 NH3 + 3 O2 → 2 N2 + 6 H2O
(Essay)
4.8/5  (37)
(37)
Balance the following equation by the oxidation-number method.
Fe2O3 + CO → Fe + CO2
(Essay)
4.8/5  (36)
(36)
When the redox reaction: NF3 + AlCl3 N2 + Cl2 + AlF3 is balanced, the correct coefficients for NF3 and Cl2, respectively, are ________.
(Multiple Choice)
4.7/5  (32)
(32)
Which reaction is the correctly balanced half reaction (in acid solution) for the process below?
Cr2O72- (aq) Cr3+ (aq)
(Multiple Choice)
4.7/5  (40)
(40)
Determine the oxidation number of the underlined element in NaBrO4.
(Multiple Choice)
4.9/5  (30)
(30)
Which of the following equations is incorrectly classified as to type of chemical reaction?
(Multiple Choice)
4.9/5  (31)
(31)
Assign an oxidation number to each atom in the following chemical reaction.
As2O3 + 5 H2O + 2 I2 ? 2 H3AsO4 + 4 HI
(Essay)
4.9/5  (44)
(44)
Write the balanced half reactions for oxidation and reduction for the following chemical equation.
Fe (s) + Cu(NO3)2 (aq) → Fe(NO3)3 (aq) + Cu (s)
(Essay)
5.0/5  (42)
(42)
How many electrons are lost or gained by each formula unit of CuBr2 in the reaction
Zn + CuBr2 ZnBr2 + Cu
(Multiple Choice)
4.9/5  (34)
(34)
During the recharging of a lead storage battery, what products(s) are formed?
(Multiple Choice)
4.7/5  (35)
(35)
Balance the following reactions for the solution type indicted. Be sure to identify which is oxidized and reduced.
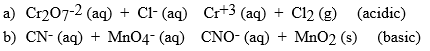
(Essay)
4.9/5  (31)
(31)
Showing 41 - 60 of 70
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)