Exam 4: Basic Concepts About Matter
Exam 1: The Science of Chemistry62 Questions
Exam 2: Numbers From Measurements99 Questions
Exam 3: Unit Systems and Dimensional Analysis83 Questions
Exam 4: Basic Concepts About Matter75 Questions
Exam 5: Atoms, Molecules, Formulas, and Subatomic Particles87 Questions
Exam 6: Electronic Structure and Chemical Periodicity92 Questions
Exam 7: Chemical Bonds92 Questions
Exam 8: Chemical Nomenclature97 Questions
Exam 9: Chemical Calculations: the Mole Concept and Chemical Formulas93 Questions
Exam 10: Chemical Calculations Involving Chemical Equations68 Questions
Exam 11: States of Matter65 Questions
Exam 12: Gas Laws78 Questions
Exam 13: Solutions64 Questions
Exam 14: Acids, Bases and Salts65 Questions
Exam 15: Oxidation and Reduction70 Questions
Exam 16: Reaction Rates and Chemical Equilibrium52 Questions
Exam 17: Nuclear Chemistry69 Questions
Select questions type
Indicate whether the following descriptions of matter represents an
-Two elements present, composition is variable. ____________________
(Multiple Choice)
4.9/5  (34)
(34)
The schematic below illustrates the breakdown of a compound via chemical means. Identify the products (as elements or compounds) formed at the end of chemical means 1 and the products formed at the end of chemical means 2.
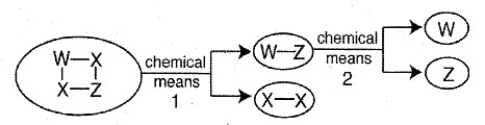
(Essay)
4.8/5  (35)
(35)
In which of the following time periods was the greatest number of elements discovered?
(Multiple Choice)
4.8/5  (40)
(40)
For each set of elements choose the appropriate characterization of the set's elemental symbols from the response list. Responses may be used more than once or need not be used at all.
- oxygen, fluorine, tungsten
(Multiple Choice)
4.8/5  (39)
(39)
For each characterization of matter, choose the appropriate classification from the response list. Responses may be used more than once or need not be used at all.
- 2 substances present, 1 phase present
(Multiple Choice)
4.9/5  (34)
(34)
If a tablespoon of table salt is mixed with a tablespoon of water, the resulting solution can be classified as a ________.
(Multiple Choice)
4.7/5  (39)
(39)
In which of the following pairs are both chemical properties?
(Multiple Choice)
4.8/5  (30)
(30)
The state of matter of a substance is determined by its ________.
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following type of matter/classification of matter pairs is incorrectly matched?
(Multiple Choice)
4.9/5  (38)
(38)
Which response contains all of the following that are physical changes and no chemical changes?
I. Candle wax melts.
II. Skin turns yellow when it comes in contact with nitric acid.
III. Milk curdles.
IV. Ice floats on water.
V. Windows fog when the temperature drops rapidly.
(Multiple Choice)
4.8/5  (29)
(29)
For each characterization of matter, choose the appropriate classification from the response list. Responses may be used more than once or need not be used at all.
- 1 substance present, 1 phase present, substance cannot be decomposed by chemical means
(Multiple Choice)
4.9/5  (33)
(33)
In which of the following sequences of elements do each of the elements have a two-letter symbol?
(Multiple Choice)
4.8/5  (46)
(46)
For each characterization of matter, choose the appropriate classification from the response list. Responses may be used more than once or need not be used at all.
- 1 substance present, 2 phases present, substance cannot be decomposed by chemical means
(Multiple Choice)
4.8/5  (30)
(30)
How many total atoms are contained in the following formula: KAl(SO4)2 ?
(Multiple Choice)
4.7/5  (39)
(39)
Which of the following statements does not describe a physical property?
(Multiple Choice)
4.9/5  (38)
(38)
Showing 41 - 60 of 75
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)