Exam 3: Unit Systems and Dimensional Analysis
Exam 1: The Science of Chemistry62 Questions
Exam 2: Numbers From Measurements99 Questions
Exam 3: Unit Systems and Dimensional Analysis83 Questions
Exam 4: Basic Concepts About Matter75 Questions
Exam 5: Atoms, Molecules, Formulas, and Subatomic Particles87 Questions
Exam 6: Electronic Structure and Chemical Periodicity92 Questions
Exam 7: Chemical Bonds92 Questions
Exam 8: Chemical Nomenclature97 Questions
Exam 9: Chemical Calculations: the Mole Concept and Chemical Formulas93 Questions
Exam 10: Chemical Calculations Involving Chemical Equations68 Questions
Exam 11: States of Matter65 Questions
Exam 12: Gas Laws78 Questions
Exam 13: Solutions64 Questions
Exam 14: Acids, Bases and Salts65 Questions
Exam 15: Oxidation and Reduction70 Questions
Exam 16: Reaction Rates and Chemical Equilibrium52 Questions
Exam 17: Nuclear Chemistry69 Questions
Select questions type
The metric length unit that is closest in size to the English unit mile is ________.
(Multiple Choice)
4.8/5  (41)
(41)
In which of the following is the metric system prefix incorrectly paired with a power of ten?
(Multiple Choice)
5.0/5  (31)
(31)
A sample of metal has a mass of 27.24 g and a volume of 2.20 mL. What is the correct value (correct number of significant figures) of its density using these data?
(Multiple Choice)
4.8/5  (45)
(45)
In which of the following is the metric system unit incorrectly paired with an abbreviation?
(Multiple Choice)
4.8/5  (28)
(28)
Using the dimensional analysis method of problem solving, calculate the velocity, in yards per second, that is equivalent to a velocity of 50.00 m/day.
(Essay)
4.8/5  (37)
(37)
An astronaut whose mass is equal to 82 kg on Earth, on the Moon will
-have the same mass on the Moon as on Earth.
(True/False)
4.7/5  (40)
(40)
Which of the following is the correct formula for calculating the volume of a sphere?
(Multiple Choice)
4.9/5  (34)
(34)
The density of osmium, the densest metal known, is 2260 cg/cm3. A rectangular block of osmium is depicted below. Calculate the mass of the block of osmium in pounds.
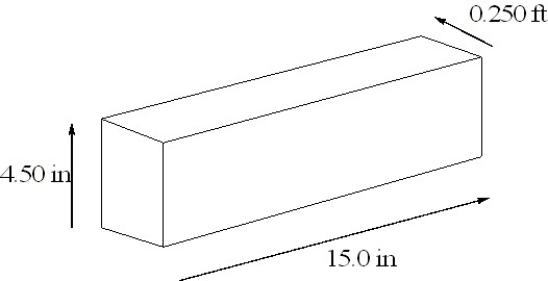
(Short Answer)
4.7/5  (38)
(38)
Chloroform was used as an anesthetic in the early days of surgery. If its density is 1.492 g/mL, what is the mass of 225 mL?
(Multiple Choice)
4.9/5  (40)
(40)
The average adult has approximately 9.0 pints of blood. If 20 drops of blood is equivalent to 1 mL, how many drops of blood does the average human contain?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following unit conversion factors would change g/mL to lb/ft3 ?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following three-dimensional shapes is not correctly paired with the formula used to calculate its volume?
(Multiple Choice)
4.8/5  (28)
(28)
A cylindrical slug of copper has a diameter of 68.3 mm and a height of 0.120 m. What is the volume of the cylinder of copper in cm3? (cylinder volume: V = pr2h)
(Multiple Choice)
4.8/5  (46)
(46)
How many grams of alcohol are present in 2 bottles (992 g total mass of mixture) of an alcohol/water mixture of that is 25.6% alcohol by mass?
(Multiple Choice)
4.8/5  (35)
(35)
A hospital patient has an oral temperature of 39.5 °C and weighs 190. lb. He is to receive a drug, the total dosage of which is 55 mg per kilogram of body weight. The drug is dissolved in water (25 mg per milliliter).
-What mass in milligrams of pure drug should he receive?
(Short Answer)
4.8/5  (33)
(33)
A hospital patient has an oral temperature of 39.5 °C and weighs 190. lb. He is to receive a drug, the total dosage of which is 55 mg per kilogram of body weight. The drug is dissolved in water (25 mg per milliliter).
-What is his weight in kilograms?
(Short Answer)
4.8/5  (32)
(32)
Which of the following unit conversion factor sequences would change milligrams to hectograms?
(Multiple Choice)
5.0/5  (40)
(40)
In which of the following "unit set-ups" are the units incorrectly combined together?
(Multiple Choice)
4.7/5  (37)
(37)
Using the dimensional analysis method of problem solving, carry out the following metric-metric conversions:
-65 cm3 = ? km3
(Essay)
4.7/5  (46)
(46)
Showing 61 - 80 of 83
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)