Exam 2: Essential Chemistry for Biology
Exam 1: Introduction: Biology Today50 Questions
Exam 2: Essential Chemistry for Biology46 Questions
Exam 3: The Molecules of Life49 Questions
Exam 4: A Tour of the Cell52 Questions
Exam 5: The Working Cell52 Questions
Exam 6: Cellular Respiration: Obtaining Energy From Food51 Questions
Exam 7: Photosynthesis: Using Light to Make Food53 Questions
Exam 8: Cellular Reproduction: Cells From Cells51 Questions
Exam 9: Patterns of Inheritance49 Questions
Exam 10: The Structure and Function of Dna53 Questions
Exam 11: How Genes Are Controlled49 Questions
Exam 12: DNA Technology50 Questions
Exam 13: How Populations Evolve50 Questions
Exam 14: How Biological Diversity Evolves46 Questions
Exam 15: The Evolution of Microbial Life58 Questions
Exam 16: The Evolution of Plants and Fungi53 Questions
Exam 17: The Evolution of Animals58 Questions
Exam 18: An Introduction to Ecology and the Biosphere53 Questions
Exam 19: Population Ecology47 Questions
Exam 20: Communities and Ecosystems52 Questions
Exam 21: Unifying Concepts of Animal Structure and Function49 Questions
Exam 22: Nutrition and Digestion57 Questions
Exam 23: Circulation and Respiration61 Questions
Exam 24: The Bodys Defenses57 Questions
Exam 25: Hormones52 Questions
Exam 26: Reproduction and Development53 Questions
Exam 27: Nervous,Sensory,and Locomotor Systems62 Questions
Exam 28: The Life of a Flowering Plant68 Questions
Exam 29: The Working Plant57 Questions
Select questions type
In the following reaction,what type of bond is holding the two atoms together? K+Cl → K⁺ + Cl− → KCl
Free
(Multiple Choice)
4.7/5  (32)
(32)
Correct Answer:
B
In order to have a positive charge,an atom must have ________.
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
A
Beryllium's atomic mass is 9,and its atomic number is 4.How many neutrons are found in a beryllium atom?
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
D
Isotopes of an element have the same number of ________ and different numbers of ________.
(Multiple Choice)
4.8/5  (36)
(36)
Please read the following scenario to answer the following questions.
While radioactive isotopes are used in medicine to identify tumors and other diseases,they can also be used to treat diseases such as cancer.One method to treat cancerous tumors is to expose them to radiation,which can kill the cancerous cells and the tumor.In 2013,the U.S.Food and Drug Administration approved a new cancer treatment based on the radioactive isotope radium-223; this isotope has a half-life of 11.4 days.The radioactive drug,known as Xofigo®,is injected into the patient's bloodstream and travels to certain regions of the body.Because it emits high-energy radiation over short distances,it can kill cancerous cells in the sites where it localizes.
After the drug was injected into a person,the following data were collected regarding the amount of radiation measured in different organs in the person's body.
Organ Measured radiation Brain 0.37 Liver 11.01 Bones 4262.60 Lungs 0.27 Urinary bladder 14.90
-A cancer patient receives an injection of the drug on March 1.Suppose that the patient must receive a second injection once the amount of the drug decreases to less than 6% in his or her body.On approximately what day should the cancer patient schedule an appointment to receive another injection?
(Multiple Choice)
4.9/5  (38)
(38)
The bond between oppositely charged ions is a(n)________ bond.
(Multiple Choice)
4.9/5  (38)
(38)
Examine the drawing of an atom below.The art is technically INCORRECT in that ________. 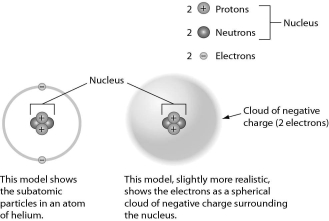
(Multiple Choice)
4.8/5  (35)
(35)
How can radiation be controlled and safely used in medicine?
(Multiple Choice)
4.9/5  (38)
(38)
How many oxygen atoms are in the products of the following reaction? C₆H₁₂O₆ + 6 H₂O + 6 O₂ → 6 CO₂ + 12 H₂O
(Multiple Choice)
4.8/5  (32)
(32)
Examine the pH scale below.How does household bleach compare to household ammonia? 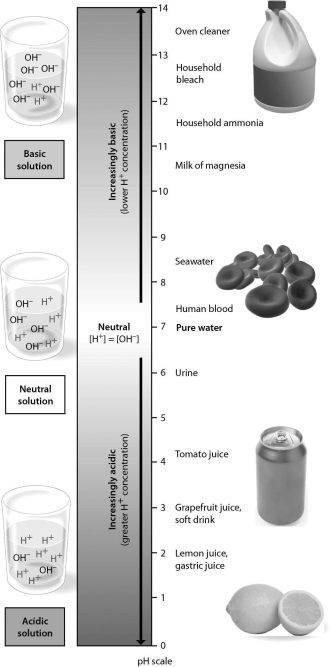
(Multiple Choice)
4.8/5  (36)
(36)
An uncharged atom of gold has an atomic number of 79 and an atomic mass of 197.This atom has ________ protons,________ neutrons,and ________ electrons.
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following elements,essential to life,is a trace element?
(Multiple Choice)
4.9/5  (31)
(31)
Why did the sweat on Heather's forehead and arms form drops?
(Multiple Choice)
4.8/5  (43)
(43)
Showing 1 - 20 of 46
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)