Exam 3: The Quantum Mechanical Model of the Atom
Exam 1: Essentials: Units, Measurements, and Problem Solving101 Questions
Exam 2: Atoms137 Questions
Exam 3: The Quantum Mechanical Model of the Atom141 Questions
Exam 4: Periodic Properties of the Elements144 Questions
Exam 5: Molecules and Compounds202 Questions
Exam 6: Chemical Bonding I138 Questions
Exam 7: Chemical Bonding Ii64 Questions
Exam 8: Chemical Reactions and Chemical Quantities79 Questions
Exam 9: Introduction to Solutions and Aqueous Reactions177 Questions
Exam 10: Thermochemistry145 Questions
Exam 11: Gases185 Questions
Exam 12: Liquids, Solids, and Intermolecular Forces132 Questions
Exam 13: Crystalline Solids and Modern Materials43 Questions
Exam 14: Solutions148 Questions
Exam 15: Chemical Kinetics155 Questions
Exam 16: Chemical Equilibrium141 Questions
Exam 17: Acids and Bases159 Questions
Exam 18: Aqueous Ionic Equilibrium188 Questions
Exam 19: Free Energy and Thermodynamics130 Questions
Exam 20: Electrochemistry148 Questions
Exam 21: Radioactivity and Nuclear Chemistry136 Questions
Exam 22: Organic Chemistry104 Questions
Exam 23: Transition Metals and Coordination Compounds74 Questions
Select questions type
How many photons are contained in a flash of green light (525 nm)that contains 189 kJ of energy?
(Multiple Choice)
4.8/5  (41)
(41)
How many different values of l are possible in the third principal level?
(Multiple Choice)
4.8/5  (35)
(35)
Determine the velocity of a medicine ball (m = 10.0 kg)with a wavelength of 1.33 × 10-35 m.
(Multiple Choice)
4.9/5  (32)
(32)
Place the following types of electromagnetic radiation in order of increasing frequency. x-rays infrared light gamma rays
(Multiple Choice)
4.8/5  (40)
(40)
Calculate the wavelength of light associated with the transition from 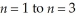 in the hydrogen atom.
in the hydrogen atom.
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following transitions (in a hydrogen atom)represent absorption of the smallest frequency photon?
(Multiple Choice)
4.7/5  (34)
(34)
In which orbital below would an electron (on average)be farthest from the nucleus?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following visible colors of light has the longest wavelength?
(Multiple Choice)
4.9/5  (37)
(37)
Calculate the energy change associated with the transition from 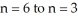 in the hydrogen atom.
in the hydrogen atom.
(Multiple Choice)
4.8/5  (26)
(26)
Determine the end (final)value of n in a hydrogen atom transition,if the electron starts in n = 2 and the atom absorbs a photon of light with a frequency of 4.57 × 1014 Hz.
(Multiple Choice)
4.7/5  (34)
(34)
The de Broglie wavelength of an electron with a velocity of 7.40 × 106 m/s is ________ m.The mass of the electron is 9.11 ×  g.
g.
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following quantum numbers describes the orientation of an orbital?
(Multiple Choice)
4.8/5  (44)
(44)
How many orbitals are contained in the third principal level (n = 3)of a given atom?
(Multiple Choice)
4.7/5  (38)
(38)
Showing 121 - 140 of 141
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)