Exam 3: The Quantum Mechanical Model of the Atom
Exam 1: Essentials: Units, Measurements, and Problem Solving101 Questions
Exam 2: Atoms137 Questions
Exam 3: The Quantum Mechanical Model of the Atom141 Questions
Exam 4: Periodic Properties of the Elements144 Questions
Exam 5: Molecules and Compounds202 Questions
Exam 6: Chemical Bonding I138 Questions
Exam 7: Chemical Bonding Ii64 Questions
Exam 8: Chemical Reactions and Chemical Quantities79 Questions
Exam 9: Introduction to Solutions and Aqueous Reactions177 Questions
Exam 10: Thermochemistry145 Questions
Exam 11: Gases185 Questions
Exam 12: Liquids, Solids, and Intermolecular Forces132 Questions
Exam 13: Crystalline Solids and Modern Materials43 Questions
Exam 14: Solutions148 Questions
Exam 15: Chemical Kinetics155 Questions
Exam 16: Chemical Equilibrium141 Questions
Exam 17: Acids and Bases159 Questions
Exam 18: Aqueous Ionic Equilibrium188 Questions
Exam 19: Free Energy and Thermodynamics130 Questions
Exam 20: Electrochemistry148 Questions
Exam 21: Radioactivity and Nuclear Chemistry136 Questions
Exam 22: Organic Chemistry104 Questions
Exam 23: Transition Metals and Coordination Compounds74 Questions
Select questions type
For hydrogen,what is the wavelength of the photon emitted when an electron drops from a 4d orbital to a 2s orbital in a hydrogen atom? The Rydberg constant is 1.097 × 10-2 nm-1.
(Multiple Choice)
4.8/5  (41)
(41)
When waves of equal amplitude from two sources are out of phase when they interact,it is called
(Multiple Choice)
4.9/5  (37)
(37)
Determine the mass of a ball with a wavelength of 3.45 × 10-34 m and a velocity of 6.55 m/s.
(Multiple Choice)
4.9/5  (41)
(41)
What is the maximum number of f orbitals that are possible in a given shell?
(Multiple Choice)
4.8/5  (35)
(35)
An FM radio station broadcasts electromagnetic radiation at a frequency of 92.6 MHz.The wavelength of this radiation is ________ m.
(Multiple Choice)
4.9/5  (32)
(32)
Calculate the frequency of the green light emitted by a hydrogen atom with a wavelength of 486.1 nm.
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following types of waves are considered electromagnetic radiation:
A.Radio Waves;
B.Sound Waves;
C.Microwaves;
D.Ocean Waves;
E.Mechanical Waves
(Multiple Choice)
4.9/5  (32)
(32)
In which orbital below would an electron (on average)be closest to the nucleus?
(Multiple Choice)
4.8/5  (33)
(33)
The number of cycles that pass through a stationary point is called
(Multiple Choice)
4.9/5  (36)
(36)
What is the wavelength of an electron (m = 9.11 × 10-28 g)moving at 1/5 the speed of light?
(Multiple Choice)
4.8/5  (36)
(36)
Why do atoms only emit certain wavelengths of light when they are excited?
(Why do line spectra exist?)
(Essay)
4.8/5  (40)
(40)
Which of the following colors of electromagnetic radiation has the shortest wavelength?
(Multiple Choice)
4.9/5  (36)
(36)
Calculate the frequency of the light emitted a photon with a wavelength of 120 nm.
(Multiple Choice)
4.8/5  (41)
(41)
It is possible to determine the ionization energy for hydrogen using the Bohr equation.Calculate the ionization energy for an atom of hydrogen,making the assumption that ionization is the transition from 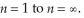
(Multiple Choice)
4.8/5  (43)
(43)
Showing 101 - 120 of 141
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)