Exam 12: Liquids, Solids, and Intermolecular Forces
Exam 1: Essentials: Units, Measurements, and Problem Solving101 Questions
Exam 2: Atoms137 Questions
Exam 3: The Quantum Mechanical Model of the Atom141 Questions
Exam 4: Periodic Properties of the Elements144 Questions
Exam 5: Molecules and Compounds202 Questions
Exam 6: Chemical Bonding I138 Questions
Exam 7: Chemical Bonding Ii64 Questions
Exam 8: Chemical Reactions and Chemical Quantities79 Questions
Exam 9: Introduction to Solutions and Aqueous Reactions177 Questions
Exam 10: Thermochemistry145 Questions
Exam 11: Gases185 Questions
Exam 12: Liquids, Solids, and Intermolecular Forces132 Questions
Exam 13: Crystalline Solids and Modern Materials43 Questions
Exam 14: Solutions148 Questions
Exam 15: Chemical Kinetics155 Questions
Exam 16: Chemical Equilibrium141 Questions
Exam 17: Acids and Bases159 Questions
Exam 18: Aqueous Ionic Equilibrium188 Questions
Exam 19: Free Energy and Thermodynamics130 Questions
Exam 20: Electrochemistry148 Questions
Exam 21: Radioactivity and Nuclear Chemistry136 Questions
Exam 22: Organic Chemistry104 Questions
Exam 23: Transition Metals and Coordination Compounds74 Questions
Select questions type
Which of the following substances would you predict to have the highest ΔHvap?
(Multiple Choice)
4.9/5  (34)
(34)
Give the term for the temperature at which the gas and liquid phases form a supercritical fluid.
(Multiple Choice)
4.8/5  (28)
(28)
Place the following substances in order of increasing boiling point. CH3CH2OH He CH3OCH3
(Multiple Choice)
4.8/5  (41)
(41)
Assign the appropriate labels to the phase diagram shown below. 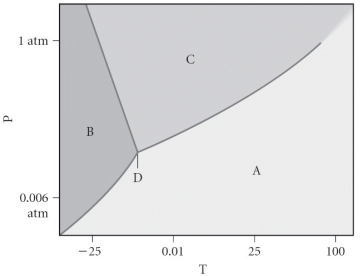
(Multiple Choice)
4.9/5  (47)
(47)
Which of the following substances would you predict to have the highest ΔHvap?
(Multiple Choice)
4.9/5  (45)
(45)
Calculate the energy that is required to change 50.0 g ice at -30.0°C to a liquid at 73.0°C.The heat of fusion = 333 J/g,the heat of vaporization = 2256 J/g,and the specific heat capacities of ice = 2.06 J /gK and liquid water = 4.184 J /gK.
(Multiple Choice)
4.8/5  (43)
(43)
How much energy is required to heat 87.1 g acetone (molar mass=58.08 g/mol)from a solid at -154.0°C to a liquid at -42.0°C? The following physical data may be useful.
ΔHfus = 7.27 kJ/mol
Cliq = 2.16 J/g°C
Cgas = 1.29 J/g°C
Csol = 1.65 J/g°C
Tmelting = -95.0°C
(Multiple Choice)
4.9/5  (44)
(44)
Place the following substances in order of decreasing boiling point. H2O N2 CO
(Multiple Choice)
4.8/5  (32)
(32)
Choose the molecule or compound that exhibits dipole-dipole forces as its strongest intermolecular force.
(Multiple Choice)
4.8/5  (36)
(36)
Choose the substance with the lowest vapor pressure at a given temperature.
(Multiple Choice)
4.8/5  (36)
(36)
Ethyl chloride,C2H5Cl,is used as a local anesthetic.It works by cooling tissue as it vaporizes; its heat of vaporization is 26.4 kJ/mol.How much heat could be removed by 20.0 g of ethyl chloride?
(Multiple Choice)
4.8/5  (44)
(44)
Determine the vapor pressure (in mm Hg)of a substance at 29°C,whose normal boiling point is 76°C and has a ΔHvap of 38.7 kJ/mol.
(Multiple Choice)
4.7/5  (42)
(42)
Choose the compound that exhibits hydrogen bonding as its strongest intermolecular force.
(Multiple Choice)
4.8/5  (38)
(38)
Showing 41 - 60 of 132
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)