Exam 12: Liquids, Solids, and Intermolecular Forces
Exam 1: Essentials: Units, Measurements, and Problem Solving101 Questions
Exam 2: Atoms137 Questions
Exam 3: The Quantum Mechanical Model of the Atom141 Questions
Exam 4: Periodic Properties of the Elements144 Questions
Exam 5: Molecules and Compounds202 Questions
Exam 6: Chemical Bonding I138 Questions
Exam 7: Chemical Bonding Ii64 Questions
Exam 8: Chemical Reactions and Chemical Quantities79 Questions
Exam 9: Introduction to Solutions and Aqueous Reactions177 Questions
Exam 10: Thermochemistry145 Questions
Exam 11: Gases185 Questions
Exam 12: Liquids, Solids, and Intermolecular Forces132 Questions
Exam 13: Crystalline Solids and Modern Materials43 Questions
Exam 14: Solutions148 Questions
Exam 15: Chemical Kinetics155 Questions
Exam 16: Chemical Equilibrium141 Questions
Exam 17: Acids and Bases159 Questions
Exam 18: Aqueous Ionic Equilibrium188 Questions
Exam 19: Free Energy and Thermodynamics130 Questions
Exam 20: Electrochemistry148 Questions
Exam 21: Radioactivity and Nuclear Chemistry136 Questions
Exam 22: Organic Chemistry104 Questions
Exam 23: Transition Metals and Coordination Compounds74 Questions
Select questions type
Identify the compound that does not have hydrogen bonding.
(Multiple Choice)
4.9/5  (47)
(47)
An unknown substance has a boiling point of 150°C at 650 torr,and a boiling point of 195°C at 780 torr.What is the heat of vaporization?
(Multiple Choice)
4.9/5  (43)
(43)
The boiling point of H2O is much higher than that of the analogous molecule H2S.This is mostly due to
(Multiple Choice)
5.0/5  (25)
(25)
Choose the pair of substances that are most likely to form a homogeneous solution.
(Multiple Choice)
4.8/5  (35)
(35)
The fluorocarbon C2CL3F3 has a normal boiling point of 47.6°C.The specific heats of C2CL3F3(1)and C2CL3F3(g)are 0.91 J/gK and 0.67 J/gK,respectively.The heat of vaporization of the compound is 27.49 kJ/mol.The heat required to convert 50.0 g of the compound from the liquid at 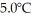 to the gas at 80.0°C is ________ kJ.
to the gas at 80.0°C is ________ kJ.
(Multiple Choice)
4.9/5  (35)
(35)
Determine the normal boiling point of a substance whose vapor pressure is 55.1 mm Hg at 35°C and has a ΔHvap of 32.1 kJ/mol.
(Multiple Choice)
4.9/5  (39)
(39)
Place the following substances in order of decreasing vapor pressure at a given temperature. PF5 BrF3 CF4
(Multiple Choice)
4.8/5  (34)
(34)
Choose the pair of substances that are most likely to form a homogeneous solution.
(Multiple Choice)
4.9/5  (38)
(38)
Identify the compound that does not have dipole-dipole forces as its strongest force.
(Multiple Choice)
4.9/5  (33)
(33)
Consider the phase diagram shown.Choose the statement below that is TRUE. 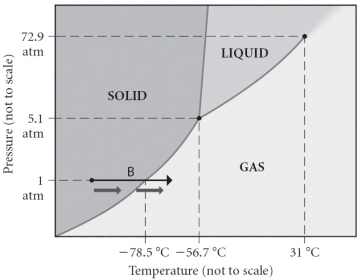
(Multiple Choice)
4.8/5  (39)
(39)
How much energy is required to vaporize 98.6 g of ethanol (C2H5OH)at its boiling point,if its ΔHvap is 40.5 kJ/mol?
(Multiple Choice)
4.8/5  (33)
(33)
Consider the phase diagram shown below.If you start at 0.75 atm and 0 °C,and move to 0.10 atm and 25C,you will move from the ________ phase to the ________ phase. 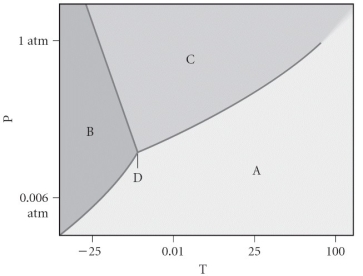
(Multiple Choice)
4.9/5  (34)
(34)
Based on the figure above,the boiling point of diethyl ether under an external pressure of 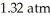 is ________ °C.
is ________ °C.
(Multiple Choice)
4.8/5  (47)
(47)
How much energy is required to vaporize 48.7 g of dichloromethane (CH2Cl2)at its boiling point,if its ΔHvap is 31.6 kJ/mol?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following compounds has dipole-dipole interactions as the strongest intermolecular force?
(Multiple Choice)
4.7/5  (33)
(33)
Showing 101 - 120 of 132
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)