Exam 17: Acids and Bases
Exam 1: Essentials: Units, Measurements, and Problem Solving101 Questions
Exam 2: Atoms137 Questions
Exam 3: The Quantum Mechanical Model of the Atom141 Questions
Exam 4: Periodic Properties of the Elements144 Questions
Exam 5: Molecules and Compounds202 Questions
Exam 6: Chemical Bonding I138 Questions
Exam 7: Chemical Bonding Ii64 Questions
Exam 8: Chemical Reactions and Chemical Quantities79 Questions
Exam 9: Introduction to Solutions and Aqueous Reactions177 Questions
Exam 10: Thermochemistry145 Questions
Exam 11: Gases185 Questions
Exam 12: Liquids, Solids, and Intermolecular Forces132 Questions
Exam 13: Crystalline Solids and Modern Materials43 Questions
Exam 14: Solutions148 Questions
Exam 15: Chemical Kinetics155 Questions
Exam 16: Chemical Equilibrium141 Questions
Exam 17: Acids and Bases159 Questions
Exam 18: Aqueous Ionic Equilibrium188 Questions
Exam 19: Free Energy and Thermodynamics130 Questions
Exam 20: Electrochemistry148 Questions
Exam 21: Radioactivity and Nuclear Chemistry136 Questions
Exam 22: Organic Chemistry104 Questions
Exam 23: Transition Metals and Coordination Compounds74 Questions
Select questions type
What is the hydronium ion concentration of a 0.150 M hypochlorous acid solution with 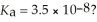 The equation for the dissociation of hypochlorous acid is:
The equation for the dissociation of hypochlorous acid is: 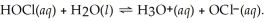
(Multiple Choice)
5.0/5  (35)
(35)
Which of the following bases is the weakest? The base is followed by its Kb value.
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following solutions would have the lowest pH? Assume that they are all 0.10 M in acid at 25∘C.The acid is followed by its Ka value.
(Multiple Choice)
4.8/5  (38)
(38)
Determine the pH of a 0.461 M C6H5CO2H M solution if the Ka of C6H5CO2H is 6.5 × 10-5.
(Multiple Choice)
4.9/5  (33)
(33)
Calculate the pH of a 0.200 M NaCN solution.The Ka for HCN is 4.9 × 10-10.
(Multiple Choice)
4.9/5  (39)
(39)
Determine the [H3O⁺] in a 0.265 M HClO solution.The Ka of HClO is 2.9 × 10-8.
(Multiple Choice)
4.8/5  (39)
(39)
Determine the pH of a 0.580 M KCH3CO2 solution at 25°C.The Ka of CH3CO2H is 1.80 × 10-5.
(Multiple Choice)
4.9/5  (46)
(46)
A solution with a hydroxide ion concentration of 4.15 × 10-6 M is ________ and has a hydrogen ion concentration of ________.
(Multiple Choice)
4.8/5  (38)
(38)
Determine the pOH of a 0.227 M C5H5N solution at 25°C.The Kb of C5H5N is 1.7 × 10-9.
(Multiple Choice)
4.8/5  (37)
(37)
If an equal number of moles of the weak acid HF and the strong base KOH are added to water,is the resulting solution acidic,basic,or neutral?
(Multiple Choice)
4.8/5  (41)
(41)
Which one of the following salts,when dissolved in water,produces the solution with the highest pH?
(Multiple Choice)
4.9/5  (31)
(31)
Showing 41 - 60 of 159
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)