Exam 32: Nuclear Physics and Nuclear Radiation
Exam 1: Introduction to Physics98 Questions
Exam 2: One-Dimensional Kinematics138 Questions
Exam 3: Vectors in Physics69 Questions
Exam 4: Two-Dimensional Kinematics51 Questions
Exam 5: Newtons Laws of Motion54 Questions
Exam 6: Applications of Newtons Laws104 Questions
Exam 7: Work and Kinetic Energy55 Questions
Exam 8: Potential Energy and Conservation of Energy62 Questions
Exam 9: Linear Momentum and Collisions114 Questions
Exam 10: Rotational Kinematics and Energy60 Questions
Exam 11: Rotational Dynamics and Static Equilibrium69 Questions
Exam 12: Gravity53 Questions
Exam 13: Oscillations About Equilibrium79 Questions
Exam 14: Waves and Sound142 Questions
Exam 15: Fluids103 Questions
Exam 16: Temperature and Heat110 Questions
Exam 17: Phases and Phase Changes93 Questions
Exam 18: The Laws of Thermodynamics90 Questions
Exam 19: Electric Charges, Forces, and Fields75 Questions
Exam 20: Electric Potential and Electric124 Questions
Exam 21: Electric Current and Direct-Current Circuits228 Questions
Exam 22: Magnetism147 Questions
Exam 23: Magnetic Flux and Faradays Law of Induction98 Questions
Exam 24: Alternating-Current Circuits72 Questions
Exam 25: Electromagnetic Waves63 Questions
Exam 26: Geometrical Optics133 Questions
Exam 27: Optical Instruments103 Questions
Exam 28: Physical Optics: Interference and Diffraction119 Questions
Exam 29: Relativity98 Questions
Exam 30: Quantum Physics88 Questions
Exam 31: Atomic Physics97 Questions
Exam 32: Nuclear Physics and Nuclear Radiation137 Questions
Select questions type
The carbon in our bodies was formed in nuclear reactions in long-dead stars.How much energy was released when the right number of 4He nuclei combined to make 12C? The mass of 4He is 3728.40 MeV/c2 and the mass of 12C is 11,177.93 MeV/c2.
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
A
When a neutron collides with a uranium-235 nucleus it can induce a variety of fission reactions.One such reaction is 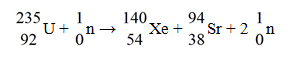 The following mass values are known:
The following mass values are known:
 What mass of uranium is needed to produce a 10-kiloton yield? (1 u = 931.5 MeV/c2 = 1.66054 × 10-27 kg,1-kiloton yield = 5.0 × 1012 J)
What mass of uranium is needed to produce a 10-kiloton yield? (1 u = 931.5 MeV/c2 = 1.66054 × 10-27 kg,1-kiloton yield = 5.0 × 1012 J)
Free
(Multiple Choice)
4.8/5  (28)
(28)
Correct Answer:
B
The half-life of a radioactive material is 4.5 days.How many days are required for a sample,with an initial activity of 1.0 × 105 Bq,to decay to an activity of 100 Bq?
(Multiple Choice)
4.8/5  (34)
(34)
One of the fusion reactions that occurs in the sun is: 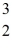 He +
He + 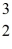 He →
He → 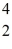 He +
He + 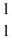 H +
H + 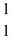 H
The following atomic masses are known:
H
The following atomic masses are known: 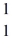 H: 1.007825 u
H: 1.007825 u 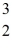 He: 3.016029 u
He: 3.016029 u 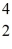 He: 4.002603 u
What is the reaction energy released in this fusion reaction? (1 u = 931.5 MeV/c2)
He: 4.002603 u
What is the reaction energy released in this fusion reaction? (1 u = 931.5 MeV/c2)
(Multiple Choice)
4.8/5  (30)
(30)
Suppose the half-life of an isotope is 2 days.You purchase 10 grams of the isotope,but it was produced in a laboratory 4 days before it was delivered to you.How much of this isotope will you have 3 days after it was delivered to you?
(Multiple Choice)
4.8/5  (44)
(44)
One material used in nuclear bombs is 239Pu,with a half-life of 24,000 years.How long must we wait for a buried stockpile of this substance to decay to 1/1000 of its original activity?
(Multiple Choice)
4.8/5  (37)
(37)
The neutral deuterium atom, 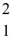 H,has a mass of 2.014102 u;a neutral ordinary hydrogen atom has a mass of 1.007825 u;a neutron has a mass of 1.008665 u;and a proton has a mass of 1.007277 u.What is the binding energy of the deuterium nucleus? (1 u = 931.5 MeV/c2)
H,has a mass of 2.014102 u;a neutral ordinary hydrogen atom has a mass of 1.007825 u;a neutron has a mass of 1.008665 u;and a proton has a mass of 1.007277 u.What is the binding energy of the deuterium nucleus? (1 u = 931.5 MeV/c2)
(Multiple Choice)
4.9/5  (33)
(33)
What happens to the half-life of a radioactive substance as it decays?
(Multiple Choice)
5.0/5  (32)
(32)
Which of the following particles (or groups of particles)are made up of quarks?
(Multiple Choice)
4.7/5  (34)
(34)
The half-life of cobalt-60 is 5.3 years,while that of strontium-90 is about 29 years.Suppose you have samples of both isotopes,and that they initially contain equal numbers of atoms of these isotopes.How will the activities (number of decays per second)of the samples compare?
(Multiple Choice)
4.9/5  (43)
(43)
A nuclear reaction is shown: 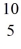 B +
B + 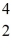 He →
He → 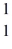 H + ? .Which one of the following isotopes is the missing nuclear product?
H + ? .Which one of the following isotopes is the missing nuclear product?
(Multiple Choice)
4.8/5  (48)
(48)
When a neutron collides with a uranium-235 nucleus it can induce a variety of fission reactions.One such reaction is 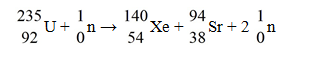 The following mass values are known:
The following mass values are known:
 How much energy is released in this reaction? (1 u = 931.5 MeV/c2)
How much energy is released in this reaction? (1 u = 931.5 MeV/c2)
(Multiple Choice)
4.9/5  (33)
(33)
In massive stars,three helium nuclei fuse together,forming a carbon nucleus,and this reaction heats the core of the star.The net mass of the three helium nuclei must therefore be
(Multiple Choice)
4.8/5  (32)
(32)
Inside the nucleus,the weakest of the four fundamental forces is
(Multiple Choice)
4.9/5  (31)
(31)
A radioactive sample has a half-life of 10 min.What fraction of the sample is left after 40 min?
(Multiple Choice)
4.9/5  (34)
(34)
When a stationary plutonium-239, 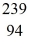 Pu,decays into uranium-235 plus an alpha particle,the energy released in the process is 5.24 MeV.The following masses are known:
Pu,decays into uranium-235 plus an alpha particle,the energy released in the process is 5.24 MeV.The following masses are known: 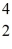 He: 4.002603 u
He: 4.002603 u 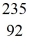 U: 235.043924 u
What is the mass of the
U: 235.043924 u
What is the mass of the 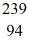 Pu nucleus,in amu? (1 u = 931.494 MeV/c2)
Pu nucleus,in amu? (1 u = 931.494 MeV/c2)
(Multiple Choice)
4.8/5  (35)
(35)
Showing 1 - 20 of 137
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)