Exam 17: Phases and Phase Changes
Exam 1: Introduction to Physics98 Questions
Exam 2: One-Dimensional Kinematics138 Questions
Exam 3: Vectors in Physics69 Questions
Exam 4: Two-Dimensional Kinematics51 Questions
Exam 5: Newtons Laws of Motion54 Questions
Exam 6: Applications of Newtons Laws104 Questions
Exam 7: Work and Kinetic Energy55 Questions
Exam 8: Potential Energy and Conservation of Energy62 Questions
Exam 9: Linear Momentum and Collisions114 Questions
Exam 10: Rotational Kinematics and Energy60 Questions
Exam 11: Rotational Dynamics and Static Equilibrium69 Questions
Exam 12: Gravity53 Questions
Exam 13: Oscillations About Equilibrium79 Questions
Exam 14: Waves and Sound142 Questions
Exam 15: Fluids103 Questions
Exam 16: Temperature and Heat110 Questions
Exam 17: Phases and Phase Changes93 Questions
Exam 18: The Laws of Thermodynamics90 Questions
Exam 19: Electric Charges, Forces, and Fields75 Questions
Exam 20: Electric Potential and Electric124 Questions
Exam 21: Electric Current and Direct-Current Circuits228 Questions
Exam 22: Magnetism147 Questions
Exam 23: Magnetic Flux and Faradays Law of Induction98 Questions
Exam 24: Alternating-Current Circuits72 Questions
Exam 25: Electromagnetic Waves63 Questions
Exam 26: Geometrical Optics133 Questions
Exam 27: Optical Instruments103 Questions
Exam 28: Physical Optics: Interference and Diffraction119 Questions
Exam 29: Relativity98 Questions
Exam 30: Quantum Physics88 Questions
Exam 31: Atomic Physics97 Questions
Exam 32: Nuclear Physics and Nuclear Radiation137 Questions
Select questions type
A metal has a latent heat of fusion of 2.32 × 104 J/kg,a specific heat of 128 J/kg ∙ K,and a melting point of 228°C.A 30-g pellet of this metal at 16°C hits a solid wall and comes to a complete stop.What would the speed of the pellet have to be in order for it to melt completely when it hits the wall,assuming that all of its kinetic energy is transformed into heat within the pellet?
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
E
A wire of diameter 0.20 mm stretches by 0.20% when a 6.28-N force is applied to it.What is Young's modulus for this wire?
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
B
The molecular weight of nitrogen,N2,is 28 g/mol.What is the rms speed of nitrogen molecules in a cooler at 8.0°C? The Boltzmann constant is 1.38 × 10-23 J/K and NA = 6.022 × 1023 molecules/mol.
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
B
If you add 700 kJ of heat to 700 g of water originally at 70.0°C,how much water is left in the container? The latent heat of vaporization of water is 22.6 × 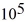 J/kg,and its specific heat capacity is
J/kg,and its specific heat capacity is 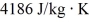 .
.
(Multiple Choice)
4.8/5  (33)
(33)
An oxygen molecule,O2,falls in a vacuum.From what height must it fall so that its translational kinetic energy at the bottom of its fall equals the average translational kinetic energy of an oxygen molecule at 920 K? The mass of one O2 molecule is 5.312 × 10-26 kg,and the Boltzmann constant is 1.38 × 10-23 J/K.Neglect air resistance and assume that g remains constant at 9.8 m/s2 throughout the fall of the molecule.
(Multiple Choice)
4.8/5  (31)
(31)
A 20.0-L pressure vessel holds 2.00 mol of oxygen at 30°C.What is the pressure inside the vessel? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.9/5  (31)
(31)
A sample of an ideal gas is heated and its Kelvin temperature doubles.If the root-mean-square speed of its molecules was originally v,what is the new root-mean-square speed?
(Multiple Choice)
4.9/5  (26)
(26)
How much heat must be removed from 456 g of water at 25.0°C to change it into ice at -10.0°C? The specific heat of ice is 2090 J/kg ∙ K,the latent heat of fusion of water is 33.5 × 104 J/kg,and the specific heat of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.8/5  (32)
(32)
A 5.3 L flask of ideal neon gas (which is monatomic)is at a pressure of 6.0 atm and a temperature of 290 K. The atomic mass of neon is 20.2 g/mol.What is the mass of the neon gas in the flask.(R = 8.31 J/mol ∙ K,1 atm = 101 kPa,NA = 6.022 × 1023 molecules/mol)
(Multiple Choice)
4.8/5  (28)
(28)
A piano wire has a radius of 0.50 mm.One end is fixed and the other is wrapped around a tuning peg,which has a diameter of 3.5 mm and is 80 cm away.After the wire just becomes taut,the peg is given three full turns.What is the tension in the wire? Young's modulus for steel is 2.0 × 1011 N/m2.
(Multiple Choice)
4.8/5  (42)
(42)
A 24.0-L tank contains ideal helium gas at 27°C and a pressure of 22.0 atm.How many moles of gas are in the tank? (R = 8.31 J/mol ∙ K,1 atm = 101 kPa)
(Multiple Choice)
4.7/5  (34)
(34)
A car starts out when the air temperature is 288 K and the absolute (total)air pressure in the tires is 500 kPa.After driving a while,the temperature of the air in the tires increases to 298 K.What is the pressure in the tires at that point,assuming their volume does not change?
(Multiple Choice)
4.8/5  (37)
(37)
What is the average translational kinetic energy of a nitrogen molecule in the air in a room in which the air temperature is 17°C? The Boltzmann constant is 1.38 × 10-23 J/K.
(Multiple Choice)
4.9/5  (34)
(34)
At what temperature would the root mean square speed of oxygen molecules,O2,be 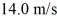 If oxygen behaves like an ideal gas? The mass of one O2 molecule is 5.312 × 10-26 kg,and the Boltzmann constant is 1.38 × 10-23 J/K.
If oxygen behaves like an ideal gas? The mass of one O2 molecule is 5.312 × 10-26 kg,and the Boltzmann constant is 1.38 × 10-23 J/K.
(Multiple Choice)
4.7/5  (36)
(36)
When a 125-kg piece of equipment is hung from a steel wire of length 75 cm,it stretches the wire by 4.00 mm.If instead the wire had a length of 150 cm,but all else were the same,by what distance would the same equipment stretch the wire?
(Multiple Choice)
4.9/5  (33)
(33)
A refrigerator has an interior volume of 0.500 m3.The temperature inside the refrigerator in 282 K,and the pressure is 101 kPa.If the molecular weight of air is 29 g/mol,what is the mass of air inside the refrigerator? (R = 8.31 J/mol × K)
(Multiple Choice)
4.9/5  (36)
(36)
A 35-g block of ice at -14°C is dropped into a calorimeter (of negligible heat capacity)containing 400 g of water at 0°C.When the system reaches equilibrium,how much ice is left in the calorimeter? The specific heat of ice is 2090 J/kg ∙ K,that of water is 4186 J/kg ∙ K,and the latent heat of fusion of water is 33.5 × 104 J/kg.
(Multiple Choice)
4.7/5  (36)
(36)
Two experimental runs are performed to determine the calorimetric properties of an alcohol which has a melting point of -10.0° C.In the first run,a 200-g cube of frozen alcohol,at the melting point,is added to 300 g of water at 20.0°C in a styrofoam container.When thermal equilibrium is reached,the alcohol-water solution is at a temperature of 5.0°C.In the second run,an identical cube of alcohol is added to 500 g of water at 20.0°C and the temperature at thermal equilibrium is 10.0°C.The specific heat capacity of water is 4190 J/kg ∙ K.Assume no heat is exchanged with the styrofoam container and the surroundings.What is the heat of fusion of the alcohol?
(Multiple Choice)
5.0/5  (33)
(33)
A 45.0-kg sample of ice is at 0.00° C.How much heat is needed to melt it? For water LF = 334,000 J/kg and LV = 2.256 × 106 J/kg.
(Multiple Choice)
4.7/5  (36)
(36)
A balloon originally has a volume of 1.0 m3 when the gas in it is at 20°C and under a pressure of 1.0 atm.As it rises in the earth's atmosphere,its volume expands.What will be its new volume if its final temperature and pressure are -40°C and 0.10 atm?
(Multiple Choice)
4.8/5  (32)
(32)
Showing 1 - 20 of 93
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)