Exam 31: Atomic Physics
Exam 1: Introduction to Physics98 Questions
Exam 2: One-Dimensional Kinematics138 Questions
Exam 3: Vectors in Physics69 Questions
Exam 4: Two-Dimensional Kinematics51 Questions
Exam 5: Newtons Laws of Motion54 Questions
Exam 6: Applications of Newtons Laws104 Questions
Exam 7: Work and Kinetic Energy55 Questions
Exam 8: Potential Energy and Conservation of Energy62 Questions
Exam 9: Linear Momentum and Collisions114 Questions
Exam 10: Rotational Kinematics and Energy60 Questions
Exam 11: Rotational Dynamics and Static Equilibrium69 Questions
Exam 12: Gravity53 Questions
Exam 13: Oscillations About Equilibrium79 Questions
Exam 14: Waves and Sound142 Questions
Exam 15: Fluids103 Questions
Exam 16: Temperature and Heat110 Questions
Exam 17: Phases and Phase Changes93 Questions
Exam 18: The Laws of Thermodynamics90 Questions
Exam 19: Electric Charges, Forces, and Fields75 Questions
Exam 20: Electric Potential and Electric124 Questions
Exam 21: Electric Current and Direct-Current Circuits228 Questions
Exam 22: Magnetism147 Questions
Exam 23: Magnetic Flux and Faradays Law of Induction98 Questions
Exam 24: Alternating-Current Circuits72 Questions
Exam 25: Electromagnetic Waves63 Questions
Exam 26: Geometrical Optics133 Questions
Exam 27: Optical Instruments103 Questions
Exam 28: Physical Optics: Interference and Diffraction119 Questions
Exam 29: Relativity98 Questions
Exam 30: Quantum Physics88 Questions
Exam 31: Atomic Physics97 Questions
Exam 32: Nuclear Physics and Nuclear Radiation137 Questions
Select questions type
The electron spin quantum number can have values of
Free
(Multiple Choice)
4.7/5  (25)
(25)
Correct Answer:
D
According to the quantum mechanical model of the hydrogen atom,if the principal quantum number is n,how many different orbital angular momentum quantum numbers are permitted?
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
B
The only valid electron state and shell designation among the following is
Free
(Multiple Choice)
4.9/5  (29)
(29)
Correct Answer:
D
The wavelength of the emitted photon if an electron in the hydrogen atom makes a transition from the n = 2 state to the ground state is closest to which of the following values? (c = 3.0 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.8/5  (32)
(32)
When an electron jumps from an orbit where n = 4 to one where n = 2
(Multiple Choice)
4.8/5  (35)
(35)
The magnetic quantum number m1 can have any integer value ranging from
(Multiple Choice)
4.7/5  (39)
(39)
In making a transition from state n = 1 to state n = 2,the hydrogen atom must
(Multiple Choice)
4.8/5  (32)
(32)
What is the value of n in the Balmer series for which the wavelength is 410.2 nm?
(Multiple Choice)
4.9/5  (31)
(31)
The only invalid electron state and shell designation among the following is
(Multiple Choice)
4.8/5  (38)
(38)
What is the wavelength of the photon emitted when an electron in a hydrogen atom which is in the initial state n = 8 jumps to the final state n = 2? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.8/5  (35)
(35)
The figure shows part of the energy level diagram of a certain atom.The energy spacing between levels 1 and 2 is twice that between 2 and 3.If an electron makes a transition from level 3 to level 2,the radiation of wavelength λ is emitted.What possible radiation wavelengths might be produced by other transitions between the three energy levels? 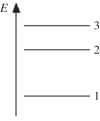
(Multiple Choice)
4.9/5  (26)
(26)
The shortest wavelength of a photon that can be emitted by a hydrogen atom,for which the initial state is 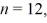 Is closest to which one of the following values?
Is closest to which one of the following values? 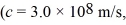
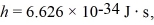
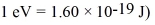
(Multiple Choice)
4.8/5  (36)
(36)
The elements in the periodic table that have completely filled shells or subshells are referred to as
(Multiple Choice)
4.9/5  (34)
(34)
The longest wavelength photon that can be emitted by a hydrogen atom,for which the final state is 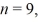 Is closest to which one of the following values? (c = 3.0 × 108 m/s,
Is closest to which one of the following values? (c = 3.0 × 108 m/s, 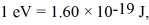
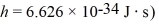
(Multiple Choice)
4.8/5  (31)
(31)
What is the electron configuration for Li,which has 3 electrons?
(Multiple Choice)
4.7/5  (20)
(20)
What is the greatest magnitude of the orbital angular momentum L that you can find in a state with 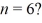
(Multiple Choice)
4.9/5  (29)
(29)
Which of the following statements are true for the Bohr model of the atom? (There could be more than one correct choice. )
(Multiple Choice)
4.9/5  (48)
(48)
In a hydrogen atom,a given electron has ℓ = 7.How many possible values can m1 have?
(Multiple Choice)
4.9/5  (27)
(27)
A hydrogen atom is in the 6h state.How many electrons are allowed in this state?
(Multiple Choice)
4.7/5  (36)
(36)
Showing 1 - 20 of 97
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)