Exam 16: Temperature and Heat
Exam 1: Introduction to Physics100 Questions
Exam 2: One-Dimensional Kinematics112 Questions
Exam 3: Vectors in Physics82 Questions
Exam 4: Two-Dimensional Kinematics95 Questions
Exam 5: Newtons Laws of Motion101 Questions
Exam 6: Applications of Newtons Laws105 Questions
Exam 7: Work and Kinetic Energy92 Questions
Exam 8: Potential Energy and Conservation of Energy99 Questions
Exam 9: Linear Momentum and Collisions102 Questions
Exam 10: Rotational Kinematics and Energy102 Questions
Exam 11: Rotational Dynamics and Static Equilibrium97 Questions
Exam 12: Gravity94 Questions
Exam 13: Oscillations About Equilibrium102 Questions
Exam 14: Waves and Sound104 Questions
Exam 15: Fluids107 Questions
Exam 16: Temperature and Heat103 Questions
Exam 17: Phases and Phase Changes100 Questions
Exam 18: The Laws of Thermodynamics97 Questions
Exam 19: Electric Charges, Forces, and Fields88 Questions
Exam 20: Electric Potential and Electric Potential Energy99 Questions
Exam 21: Electric Current and Direct-Current Circuits99 Questions
Exam 22: Magnetism101 Questions
Exam 23: Magnetic Flux and Faradays Law of Induction99 Questions
Exam 24: Alternating-Current Circuits93 Questions
Exam 25: Electromagnetic Waves90 Questions
Exam 26: Geometrical Optics92 Questions
Exam 27: Optical Instruments102 Questions
Exam 28: Physical Optics: Interference and Diffraction93 Questions
Exam 29: Relativity100 Questions
Exam 30: Quantum Physics100 Questions
Exam 31: Atomic Physics75 Questions
Exam 32: Nuclear Physics and Nuclear Radiation89 Questions
Select questions type
During a strenuous period of extravehicular activity, an astronaut generates 2.0 MJ of heat per hour, which must be removed by means of the liquid cooling and ventilating garment (LCVG) worn under the spacesuit. How much water must be circulated in the LCVG to remove this quantity of heat if it takes it from the astronaut at 37°C and dumps it at 0°C?
Free
(Multiple Choice)
4.9/5  (30)
(30)
Correct Answer:
C
Suppose that an aluminum wire were to be strung out in a loop that just fits snugly around the equator (assuming a perfectly spherical Earth with a radius of 6.37 × 106 m). If the temperature of the wire is increased by 0.50°C, and the increase in length is distributed equally through the entire length, how far off the ground will the wire loop be? The coefficient of linear expansion of aluminum is 24 × 10-6 K-1.
Free
(Multiple Choice)
4.8/5  (28)
(28)
Correct Answer:
D
The type of heat transfer that occurs between the radiator of a car and the atmosphere, when the car is in motion, is principally
(Multiple Choice)
4.9/5  (44)
(44)
FIGURE 16-3 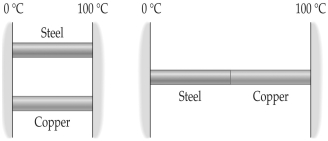 -If the absolute temperature of an object is tripled, the thermal power radiated per unit surface area of this object will (assuming that its emissivity is not affected by the temperature change)
-If the absolute temperature of an object is tripled, the thermal power radiated per unit surface area of this object will (assuming that its emissivity is not affected by the temperature change)
(Multiple Choice)
5.0/5  (42)
(42)
The coefficient of linear expansion of lead is 29 × 10-6 K-1. What change in temperature will cause a 10-m long lead bar to change in length by 3.0 mm?
(Multiple Choice)
4.8/5  (33)
(33)
0.45 kg of a metal at 90.°C is added to 0.40 kg of water at 20.0°C.
(a) If the final temperature of the mixture is 26.°C, what is the specific heat of the metal?
(b) If the water container has a significant heat capacity, will this increase or decrease the metal specific heat determined in part (a) given the same data?
(Short Answer)
5.0/5  (36)
(36)
The coefficient of linear expansion of copper is 17 × 10-6 K-1 and that of steel is 12 × 10-6 K-1. At 12°C a steel rod has a diameter of 2.540 cm and a copper pipe has a diameter of 2.536 cm. If they are heated together to a higher temperature, at what temperature will the rod fit snugly in the pipe?
(Multiple Choice)
4.8/5  (31)
(31)
At room temperature, a typical person loses energy to the surroundings at the rate of 62.0 W. If this energy loss has to be made up by an equivalent food intake, how many Calories does this person need to consume every day just to make up this heat loss?
(Multiple Choice)
5.0/5  (40)
(40)
FIGURE 16-3  -If the absolute temperature of an object is doubled, the thermal power radiated per unit surface area of this object will (assuming that its emissivity is not affected by the temperature change)
-If the absolute temperature of an object is doubled, the thermal power radiated per unit surface area of this object will (assuming that its emissivity is not affected by the temperature change)
(Multiple Choice)
5.0/5  (32)
(32)
The coefficient of linear expansion of copper is 17 × 10-6 K-1. A block of copper 30 cm wide, 45 cm long, and 10 cm thick is heated from 0°C to 100°C What is the change in the volume of the block?
(Multiple Choice)
4.9/5  (28)
(28)
Object 1 has three times the specific heat capacity and four times the mass of Object 2. The two objects are heated from the same initial temperature, T0, to the same final temperature Tf. You determine that the amount of heat gained by Object 1 is Q. The amount of heat absorbed by Object 2 will be
(Multiple Choice)
4.7/5  (41)
(41)
The weather outside is frightful. The temperature is -22°F. What is the corresponding temperature in the Celsius scale?
(Multiple Choice)
5.0/5  (38)
(38)
What is the net power that a person with surface area of 1.20 m2 radiates if his emissivity is 0.895, his skin temperature is 300 K, and he is in a room that is at a temperature of 290 K? The Stefan-Boltzmann constant is 5.67 x 10-8 W/(m2·K4).
(Multiple Choice)
4.8/5  (39)
(39)
Gas in a constant-volume gas thermometer registers a pressure of 75.0 kPa at 0°C. Assuming ideal behavior, what is the pressure of this gas at 200°C?
(Multiple Choice)
4.8/5  (29)
(29)
Heat is energy that is transferred between objects that are in thermal contact because of a temperature difference.
(True/False)
4.8/5  (30)
(30)
At what rate is the human body radiating energy when it is at 33°C?
Take the body surface area to be 1.4 m2, and approximate the body as a black body.
(Short Answer)
4.7/5  (30)
(30)
When you sit by a campfire, by what method of heat transfer are you primarily being warmed?
(Short Answer)
4.7/5  (42)
(42)
Object 1 has three times the specific heat capacity and four times the mass of Object 2. The two objects are given the same amount of heat. If the temperature of Object 1 changes by an amount ΔT, the change in temperature of Object 2 will be
(Multiple Choice)
4.8/5  (33)
(33)
Does it make sense to say that an object has twice as much heat as another?
Why?
(Essay)
4.9/5  (36)
(36)
Showing 1 - 20 of 103
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)