Exam 11: Intermolecular Forces and Liquids and Solids
Exam 1: Chemistry: The Study of Change175 Questions
Exam 2: Atoms Molecules and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions192 Questions
Exam 5: Gases130 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts136 Questions
Exam 10: Chemical Bonding II: Molecular Geometry and Hybridization of Atomic146 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics131 Questions
Exam 14: Chemical Equilibrium119 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria145 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry128 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metals Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry66 Questions
Exam 25: Synthetic and Natural Organic Polymers46 Questions
Select questions type
Based on the phase diagram shown below, how will the melting point of the substance change if the pressure is increased above 1 atm 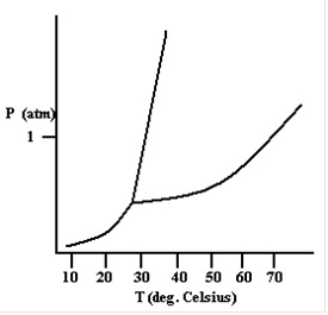
(Multiple Choice)
4.9/5  (43)
(43)
Suppose the atoms in a two-dimensional crystal have the following arrangement: 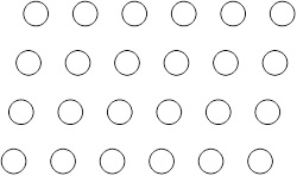 How many atoms are in one unit cell
A) One
B) Two
C) Three
D) Four
E) None of the above
How many atoms are in one unit cell
A) One
B) Two
C) Three
D) Four
E) None of the above
(True/False)
4.7/5  (27)
(27)
Which of following can form hydrogen bonds with water molecules
(1) Na+ (2) CH3COOH (3) C2H6 (4) CH3NH2
(Multiple Choice)
4.8/5  (34)
(34)
All intermolecular forces must be overcome in order for a substance to undergo a phase change from a solid to a liquid.
(True/False)
4.8/5  (25)
(25)
Calculate the amount of enthalpy required to heat 25.0 g of solid benzene (C6H6) at -10 C to liquid benzene at 20.0 C. Thermodynamic data for benzene: specific heat of solid benzene = 1.52 J/g· C; specific heat of liquid benzene = 1.73 J/g· C; melting point = 5.5 C; Hfus = 9.9 kJ/mol.
(Multiple Choice)
4.9/5  (40)
(40)
MgO has the same crystal structure as NaCl, face-centered cubic. How many oxide ions surround each Mg2+ ion as nearest neighbors
(Multiple Choice)
4.9/5  (38)
(38)
Which one of the following crystallizes in a metallic lattice
(Multiple Choice)
4.7/5  (34)
(34)
The molar enthalpy of vaporization of carbon disulfide is 26.74 kJ/mol, and its normal boiling point is 46 C. What is the vapor pressure of CS2 at 0 C
(Multiple Choice)
4.8/5  (43)
(43)
Indicate all the types of intermolecular forces of attraction in SO2(l).
(Multiple Choice)
4.9/5  (33)
(33)
The vapor pressure of a liquid in a closed container depends upon
(Multiple Choice)
4.8/5  (37)
(37)
Calculate the amount of heat that must be absorbed by 10.0 g of ice at -20 C to convert it to liquid water at 60.0 C. Given: specific heat (ice) = 2.1 J/g· C; specific heat (water) = 4.18 J/g· C; Hfus = 6.0 kJ/mol.
(Multiple Choice)
4.8/5  (34)
(34)
Indicate all the types of intermolecular forces of attraction in SF4(g).
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following would be expected to have the highest vapor pressure at room temperature
(Multiple Choice)
4.9/5  (36)
(36)
Which one of the following substances is expected to have the highest boiling point
(Multiple Choice)
4.8/5  (45)
(45)
Iron crystallizes in a body-centered cubic unit. The edge of this cell is 287 pm. What is the density of iron
(Multiple Choice)
4.8/5  (36)
(36)
BaCl2 crystallizes such that the Ba2+ ions are in a face-centered cubic arrangement and the Cl- ions are in the holes of the lattice (fluorite structure). How many Cl- ions are present in one unit cell of this crystal
(Multiple Choice)
4.8/5  (30)
(30)
Suppose the atoms in a two-dimensional crystal have the following arrangement: 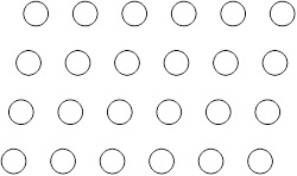 What is the coordination number of each atom in this crystal
What is the coordination number of each atom in this crystal
(Multiple Choice)
4.7/5  (34)
(34)
Showing 21 - 40 of 149
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)