Exam 11: Intermolecular Forces and Liquids and Solids
Exam 1: Chemistry: The Study of Change175 Questions
Exam 2: Atoms Molecules and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions192 Questions
Exam 5: Gases130 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts136 Questions
Exam 10: Chemical Bonding II: Molecular Geometry and Hybridization of Atomic146 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics131 Questions
Exam 14: Chemical Equilibrium119 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria145 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry128 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metals Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry66 Questions
Exam 25: Synthetic and Natural Organic Polymers46 Questions
Select questions type
The number of nearest neighbors (atoms that make contact) around each atom in a face-centered cubic lattice of a metal is
(Multiple Choice)
4.8/5  (35)
(35)
The structural form of the element Ge closely resembles the structure of
(Multiple Choice)
4.8/5  (33)
(33)
Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.0 g/cm3 at 27 C. Calculate the atomic radius of Pd.
(Multiple Choice)
4.8/5  (35)
(35)
Butter melts over a range of temperatures, rather than with a sharp melting point. Butter is classified as a/an
(Multiple Choice)
4.8/5  (29)
(29)
Ethanol (C2H5 - OH) will have a greater viscosity than ethylene glycol (HO - CH2CH2 - OH) at the same temperature.
(True/False)
5.0/5  (34)
(34)
The molar enthalpy of vaporization of boron tribromide is 30.5 kJ/mol, and its normal boiling point is 91 C. What is the vapor pressure of BBr3 at 20 C
(Multiple Choice)
4.8/5  (35)
(35)
Given the following liquids and their boiling points, which has the highest vapor pressure at its normal boiling point
(Multiple Choice)
4.8/5  (36)
(36)
Use the graph of vapor pressure to determine the normal boiling point of O2. 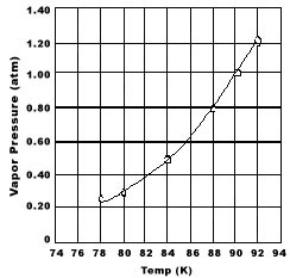
(Multiple Choice)
4.9/5  (42)
(42)
The shape of the water-to-glass meniscus results from the strong adhesive forces between glass and water.
(True/False)
4.8/5  (34)
(34)
The normal boiling point of bromine is 58.8 C. Given that the vapor pressure of bromine is 75.0 torr at 2.5 C, calculate the molar enthalpy of vaporization of bromine.
(Multiple Choice)
4.8/5  (29)
(29)
Which one of the following substances will have both dispersion forces and dipole-dipole forces
(Multiple Choice)
4.9/5  (34)
(34)
The intermolecular forces present in C6H6 include which of the following
I. dipole-dipole
II. ion-dipole
III. dispersion
IV. hydrogen bonding
(Multiple Choice)
4.9/5  (29)
(29)
At a given temperature CCl4 has a weaker surface tension than H2O.
(True/False)
4.9/5  (36)
(36)
Indicate all the types of intermolecular forces of attraction in CH3OH(l).
(Multiple Choice)
4.8/5  (39)
(39)
Showing 81 - 100 of 149
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)