Exam 11: Intermolecular Forces and Liquids and Solids
Exam 1: Chemistry: The Study of Change175 Questions
Exam 2: Atoms Molecules and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions192 Questions
Exam 5: Gases130 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts136 Questions
Exam 10: Chemical Bonding II: Molecular Geometry and Hybridization of Atomic146 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics131 Questions
Exam 14: Chemical Equilibrium119 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria145 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry128 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metals Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry66 Questions
Exam 25: Synthetic and Natural Organic Polymers46 Questions
Select questions type
A face-centered cubic unit cell is the repeating unit in which type of crystal packing
(Multiple Choice)
4.9/5  (37)
(37)
Of the following, which is the dominant (strongest) type of intermolecular force present in RbCl(s)
(Multiple Choice)
4.8/5  (34)
(34)
Indicate all the types of intermolecular forces of attraction in HCl(g).
(Multiple Choice)
4.9/5  (40)
(40)
Suppose the atoms in a two-dimensional crystal have the following arrangement: 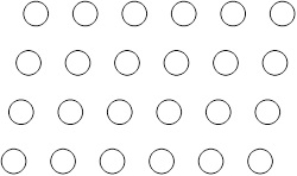 On the drawing below the lines connect four spheres which represents the unit cell of this crystal.
On the drawing below the lines connect four spheres which represents the unit cell of this crystal. 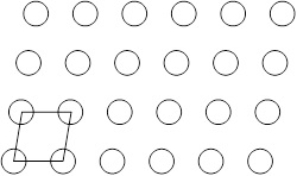
(True/False)
4.8/5  (32)
(32)
Identify the dominant (strongest) type of intermolecular force present in H2S(g).
(Multiple Choice)
4.8/5  (40)
(40)
The vapor pressure of ethanol is 400 mmHg at 63.5 C. Its molar heat of vaporization is 39.3 kJ/mol. What is the vapor pressure of ethanol, in mmHg, at 34.9 C
(Multiple Choice)
4.8/5  (35)
(35)
The most space efficient arrangement of spheres is found in which type of unit cell
(Multiple Choice)
4.8/5  (30)
(30)
Which one of the following substances is expected to have the lowest melting point
(Multiple Choice)
4.7/5  (34)
(34)
Showing 141 - 149 of 149
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)