Exam 2: Atoms, Molecules, and Ions
Exam 1: Introduction153 Questions
Exam 2: Atoms, Molecules, and Ions141 Questions
Exam 3: Stoichiometry168 Questions
Exam 4: Reactions in Aqueous Solution156 Questions
Exam 5: Gases109 Questions
Exam 6: Energy Relationships in Chemical Reactions105 Questions
Exam 7: The Electronic Structure of Atoms115 Questions
Exam 8: The Periodic Table119 Questions
Exam 9: Chemical Bonding I: the Covalent Bond118 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals120 Questions
Exam 11: Introduction to Organic Chemistry57 Questions
Exam 12: Intermolecular Forces and Liquids and Solids138 Questions
Exam 13: Physical Properties of Solutions109 Questions
Exam 14: Chemical Kinetics114 Questions
Exam 15: Chemical Equilibrium99 Questions
Exam 16: Acids and Bases163 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria92 Questions
Exam 18: Thermodynamics112 Questions
Exam 19: Redox Reactions and Electrochemistry138 Questions
Exam 20: The Chemistry of Coordination Compounds76 Questions
Exam 21: Nuclear Chemistry112 Questions
Exam 22: Organic Polymerssynthetic and Natural42 Questions
Select questions type
Use the periodic table above to show where the noble gases are located.
(Short Answer)
4.8/5  (32)
(32)
Use the periodic table above to show where the nonmetals are located.
(Short Answer)
4.8/5  (40)
(40)
What is the total number of atomic particles (protons, neutrons, and electrons)in an atom of 18F?
(Short Answer)
4.8/5  (39)
(39)
What is the formula for the binary compound formed by potassium and nitrogen?
(Multiple Choice)
5.0/5  (43)
(43)
The chemical name for ClO3- is chlorate ion. Therefore, the name of HClO3 is
(Multiple Choice)
4.9/5  (36)
(36)
Which pair of elements would be most likely to form an ionic compound?
(Multiple Choice)
4.8/5  (33)
(33)
Use the periodic table above to show where the halogen elements are located.
(Short Answer)
4.7/5  (47)
(47)
Name the binary compound formed between barium and phosphorus.
(Multiple Choice)
4.8/5  (29)
(29)
How many electrons, protons, and neutrons are in a neutral atom of the following isotope of calcium? 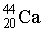
(Short Answer)
4.9/5  (36)
(36)
Write the names and symbols of two metals and two nonmetals. Identify which are the metals and which are the nonmetals.
(Essay)
4.9/5  (40)
(40)
Using a cathode ray tube, J. J. Thomson determined the magnitude of the electric charge on the electron.
(True/False)
4.7/5  (35)
(35)
Showing 81 - 100 of 141
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)