Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals
Exam 1: Introduction153 Questions
Exam 2: Atoms, Molecules, and Ions141 Questions
Exam 3: Stoichiometry168 Questions
Exam 4: Reactions in Aqueous Solution156 Questions
Exam 5: Gases109 Questions
Exam 6: Energy Relationships in Chemical Reactions105 Questions
Exam 7: The Electronic Structure of Atoms115 Questions
Exam 8: The Periodic Table119 Questions
Exam 9: Chemical Bonding I: the Covalent Bond118 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals120 Questions
Exam 11: Introduction to Organic Chemistry57 Questions
Exam 12: Intermolecular Forces and Liquids and Solids138 Questions
Exam 13: Physical Properties of Solutions109 Questions
Exam 14: Chemical Kinetics114 Questions
Exam 15: Chemical Equilibrium99 Questions
Exam 16: Acids and Bases163 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria92 Questions
Exam 18: Thermodynamics112 Questions
Exam 19: Redox Reactions and Electrochemistry138 Questions
Exam 20: The Chemistry of Coordination Compounds76 Questions
Exam 21: Nuclear Chemistry112 Questions
Exam 22: Organic Polymerssynthetic and Natural42 Questions
Select questions type
N,N-diethyl-m-tolumide (DEET)is the active ingredient in many mosquito repellents. 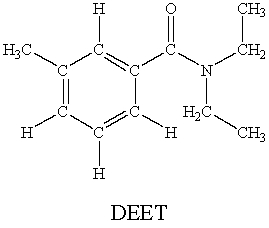 How many sigma bonds and pi bonds are contained in a DEET molecule?
How many sigma bonds and pi bonds are contained in a DEET molecule?
(Short Answer)
4.9/5  (33)
(33)
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. What is the hybridization state of carbon indicated by the arrow in the structure of ibuprofen shown below? 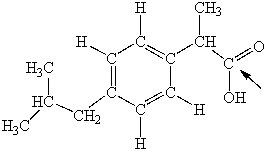
(Multiple Choice)
4.9/5  (37)
(37)
In which of the following would the bonding be weakened with the addition of an electron to form the negative molecular ion?
(Multiple Choice)
4.8/5  (43)
(43)
Give the number of lone pairs around the central atom and the geometry of the ion PCl4-.
(Multiple Choice)
4.8/5  (45)
(45)
Give the number of lone pairs around the central atom and the geometry of the ion NO2-.
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following correctly lists species in order of increasing bond order?
(Multiple Choice)
4.7/5  (35)
(35)
Which of the following species has the largest dipole moment (i.e., is the most polar)?
(Multiple Choice)
4.8/5  (46)
(46)
Give the number of lone pairs around the central atom and the molecular geometry of SeF4.
(Multiple Choice)
4.7/5  (47)
(47)
Draw a Lewis structure for PF5 that shows the correct atom arrangement predicted by VSEPR theory.
(Essay)
4.8/5  (36)
(36)
Indicate the type of hybrid orbitals used by the central atom in CCl4.
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following substances is/are bent?
(i)H2S
(ii)CO2
(iii)ClNO
(iv)NH2-
(v)O3
(Multiple Choice)
4.8/5  (48)
(48)
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. What is the hybridization state of carbon indicated by the arrow in the structure of ibuprofen shown below? 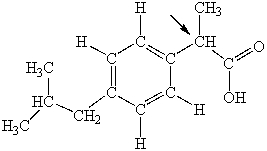
(Multiple Choice)
4.8/5  (39)
(39)
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. 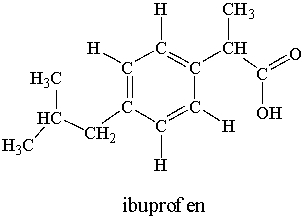 How many sigma bonds and pi bonds are contained in an ibuprofen molecule?
How many sigma bonds and pi bonds are contained in an ibuprofen molecule?
(Short Answer)
4.9/5  (41)
(41)
Give the number of lone pairs around the central atom and the geometry of the ion ClO2-.
(Multiple Choice)
4.9/5  (40)
(40)
Showing 21 - 40 of 120
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)